Abstract
Purpose
Venetoclax is a selective BCL-2 inhibitor indicated for the treatment of patients with chronic lymphocytic leukemia (CLL). It is predominately metabolized by cytochrome P450 (CYP) 3A. The study objective was to determine the effect of different dosage regimens of ritonavir, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax in 20 healthy subjects.
Methods
In cohorts 1 and 2, subjects received single 10 mg doses of venetoclax in periods 1 and 2 and a single 50- or 100-mg dose of ritonavir in period 2. In cohort 3, subjects received 10-mg venetoclax doses on day 1 of period 1 and days 1 and 11 of period 2, and 50 mg ritonavir daily on days 1 to 14 of period 2.
Results
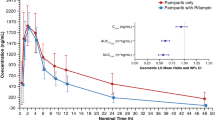
Single doses of 50 and 100 mg ritonavir increased the venetoclax maximum concentration (Cmax) 2.3- to 2.4-fold compared to venetoclax alone and the area under the curve (AUC) 6.1- and 8.1-fold, respectively. Daily 50 mg ritonavir resulted in a 2.4- and 7.9-fold increase in venetoclax Cmax and AUC, respectively. Administration of 50 mg ritonavir daily saturated CYP3A inhibition and completely inhibited the formation of the major venetoclax metabolite M27. Time-dependent CYP3A inhibition with daily 50 mg ritonavir was offset by ritonavir CYP3A induction, resulting in a limited net increase in CYP3A inhibition with multiple doses.
Conclusion
After completion of the dose ramp-up, venetoclax dose reductions of at least 75% are recommended when administered concomitantly with strong CYP3A inhibitors to maintain venetoclax exposures within the established therapeutic window for CLL treatment.



Similar content being viewed by others
References
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208. https://doi.org/10.1038/nm.3048
Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S, Kipps TJ, Anderson MA, Salem AH, Dunbar M, Zhu M, Peale F, Ross JA, Gressick L, Desai M, Kim SY, Verdugo M, Humerickhouse RA, Gordon GB, Gerecitano JF (2017) Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 35(8):826–833. https://doi.org/10.1200/jco.2016.70.4320
Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, Touzeau C, Punnoose EA, Cordero J, Munasinghe W, Jia J, Salem AH, Freise KJ, Leverson JD, Enschede SH, Ross JA, Maciag PC, Verdugo M, Harrison SJ (2017) Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 130(22):2392–2400. https://doi.org/10.1182/blood-2017-06-788323
Jones JA, Mato AR, Wierda WG, Davids MS, Choi M, Cheson BD, Furman RR, Lamanna N, Barr PM, Zhou L, Chyla B, Salem AH, Verdugo M, Humerickhouse RA, Potluri J, Coutre S, Woyach J, Byrd JC (2017) Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. pii: S1470-2045(17)30909-9. https://doi.org/10.1016/S1470-2045(17)30909-9
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374(4):311–322. https://doi.org/10.1056/NEJMoa1513257
Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A (2016) Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 6(10):1106–1117. https://doi.org/10.1158/2159-8290.CD-16-0313
Le Gouill S, Wermke M, Morschhauser F, Lim ST, Salles G, Kloos I, de Burgat V, Becquart M, Paux G, Kraus-Berthier L, Pennaforte S, Stilgenbauer S, Walewski J, Ribrag V (2017) A new BCL-2 inhibitor (S55746/BCL201) as monotherapy in patients with relapsed or refractory non-Hodgkin lymphoma: preliminary results of the first-in-human study. Hematol Oncol 35(S2):47–48
Salem AH, Agarwal SK, Dunbar M, Enschede SL, Humerickhouse RA, Wong SL (2017) Pharmacokinetics of venetoclax, a novel BCL-2 inhibitor, in patients with relapsed or refractory chronic lymphocytic leukemia or non-Hodgkin lymphoma. J Clin Pharmacol 57(4):484–492. https://doi.org/10.1002/jcph.821
Kikuchi R, Shebley M, Bow D, Carr R, Nijsen M, de Morais S (2016) In vitro characterization of drug metabolizing enzymes and transporters to enable a mechanistic drug-drug interaction assessment for venetoclax. In: 11th International ISSX Meeting, June 12-16, 2016, Busan, Korea. https://issx.confex.com/issx/intl11/webprogram/Paper36597.html
Liu H, Michmerhuizen MJ, Lao Y, Wan K, Salem AH, Sawicki J, Serby M, Vaidyanathan S, Wong SL, Agarwal S, Dunbar M, Sydor J, de Morais SM, Lee AJ (2017) Metabolism and disposition of a novel B-cell lymphoma-2 inhibitor venetoclax in humans and characterization of its unusual metabolites. Drug Metab Dispos 45(3):294–305. https://doi.org/10.1124/dmd.116.071613
Chiney MS, Menon RM, Bueno OF, Tong B, Salem AH (2017) Clinical evaluation of P-glycoprotein inhibition by venetoclax: a drug interaction study with digoxin. Xenobiotica. https://doi.org/10.1080/00498254.2017.1381779
Cheung TT, Salem AH, Menon RM, Munasinghe WP, Bueno OF, Agarwal SK (2017) Pharmacokinetics of the BCL-2 inhibitor venetoclax in healthy Chinese subjects. Clin Pharmacol Drug Dev. https://doi.org/10.1002/cpdd.395
Salem AH, Agarwal SK, Dunbar M, Nuthalapati S, Chien D, Freise KJ, Wong SL (2016) Effect of low- and high-fat meals on the pharmacokinetics of venetoclax, a selective first-in-class BCL-2 inhibitor. J Clin Pharmacol 56(11):1355–1361. https://doi.org/10.1002/jcph.741
Jones AK, Freise KJ, Agarwal SK, Humerickhouse RA, Wong SL, Salem AH (2016) Clinical predictors of venetoclax pharmacokinetics in chronic lymphocytic leukemia and non-Hodgkin’s lymphoma patients: a pooled population pharmacokinetic analysis. AAPS J 18(5):1192–1202. https://doi.org/10.1208/s12248-016-9927-9
Agarwal SK, DiNardo CD, Potluri J, Dunbar M, Kantarjian HM, Humerickhouse RA, Wong SL, Menon RM, Konopleva MY, Salem AH (2017) Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther 39(2):359–367. https://doi.org/10.1016/j.clinthera.2017.01.003
Agarwal SK, Salem AH, Danilov AV, Hu B, Puvvada S, Gutierrez M, Chien D, Lewis LD, Wong SL (2017) Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br J Clin Pharmacol 83(4):846–854. https://doi.org/10.1111/bcp.13175
Agarwal SK, Hu B, Chien D, Wong SL, Salem AH (2016) Evaluation of rifampin’s transporter inhibitory and CYP3A inductive effects on the pharmacokinetics of venetoclax, a BCL-2 inhibitor: results of a single- and multiple-dose study. J Clin Pharmacol 56(11):1335–1343. https://doi.org/10.1002/jcph.730
Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J (1999) HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol 57(10):1147–1152. https://doi.org/10.1016/S0006-2952(99)00026-X
Eichbaum C, Cortese M, Blank A, Burhenne J, Mikus G (2013) Concentration effect relationship of CYP3A inhibition by ritonavir in humans. Eur J Clin Pharmacol 69(10):1795–1800. https://doi.org/10.1007/s00228-013-1530-8
Greenblatt DJ, Harmatz JS (2015) Ritonavir is the best alternative to ketoconazole as an index inhibitor of cytochrome P450-3A in drug-drug interaction studies. Br J Clin Pharmacol 80(3):342–350. https://doi.org/10.1111/bcp.12668
Grogg KL, Miller RF, Dogan A (2007) HIV infection and lymphoma. J Clin Pathol 60(12):1365–1372. https://doi.org/10.1136/jcp.2007.051953
Salem AH, Dunbar M, Agarwal SK (2017) Pharmacokinetics of venetoclax in patients with 17p deletion chronic lymphocytic leukemia. Anti-Cancer Drugs 28(8):911–914. https://doi.org/10.1097/CAD.0000000000000522
Salem AH, Hu B, Freise KJ, Agarwal SK, Sidhu DS, Wong SL (2017) Evaluation of the pharmacokinetic interaction between venetoclax, a selective BCL-2 inhibitor, and warfarin in healthy volunteers. Clin Drug Investig 37(3):303–309. https://doi.org/10.1007/s40261-016-0485-9
Myasein F, Kim E, Zhang J, Wu H, El-Shourbagy TA (2009) Rapid, simultaneous determination of lopinavir and ritonavir in human plasma by stacking protein precipitations and salting-out assisted liquid/liquid extraction, and ultrafast LC-MS/MS. Anal Chim Acta 651(1):112–116. https://doi.org/10.1016/j.aca.2009.08.010
Freise KJ, Shebley M, Salem AH (2017) Quantitative prediction of the effect of CYP3A inhibitors and inducers on venetoclax pharmacokinetics using a physiologically based pharmacokinetic model. J Clin Pharmacol 57(6):796–804. https://doi.org/10.1002/jcph.858
Freise KJ, Jones AK, Eckert D, Mensing S, Wong SL, Humerickhouse RA, Awni WM, Salem AH (2017) Impact of venetoclax exposure on clinical efficacy and safety in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Pharmacokinet 56(5):515–523. https://doi.org/10.1007/s40262-016-0453-9
Freise KJ, Dunbar M, Jones AK, Hoffman D, Enschede SL, Wong S, Salem AH (2016) Venetoclax does not prolong the QT interval in patients with hematological malignancies: an exposure-response analysis. Cancer Chemother Pharmacol 78(4):847–853. https://doi.org/10.1007/s00280-016-3144-1
Parikh A, Gopalakrishnan S, Freise KJ, Verdugo ME, Menon RM, Mensing S, Salem AH (2017) Exposure-response evaluations of venetoclax efficacy and safety in patients with non-Hodgkin lymphoma. Leuk Lymphoma. https://doi.org/10.1080/10428194.2017.1361024
Freise KJ, Jones AK, Menon RM, Verdugo ME, Humerickhouse RA, Awni WM, Salem AH (2017) Relationship between venetoclax exposure, rituximab coadministration, and progression-free survival in patients with relapsed or refractory chronic lymphocytic leukemia: demonstration of synergy. Hematol Oncol 35(4):679–684. https://doi.org/10.1002/hon.2373
Wei A, Strickland SA, Roboz GJ, Hou J-Z, Fiedler W, Lin TL, Martinelli G, Walter RB, Enjeti A, Fakouhi K, Darden DE, Dunbar M, Zhu M, Agarwal S, Salem AH, Mabry M, Hayslip J (2016) Safety and efficacy of venetoclax plus low-dose cytarabine in treatment-naive patients aged ≥ 65 years with acute myeloid leukemia. Blood 128(22):102–102
Freise KJ, Jones AK, Verdugo ME, Menon RM, Maciag PC, Salem AH (2017) Moving beyond maximum tolerated dose for targeted oncology drugs: use of clinical utility index to optimize venetoclax dosage in multiple myeloma patients. Clin Pharmacol Ther 102(6):970–976. https://doi.org/10.1002/cpt.712
Ng J, Klein CE, Chiu YL, Awni WM, Morris JB, Podsadecki TJ, Cui Y, Bernstein B, Kim D (2008) The effect of food on ritonavir bioavailability following administration of ritonavir 100 mg film-coated tablet in healthy adult subjects. J Int AIDS Soc 11(Suppl 1):P247. https://doi.org/10.1186/1758-2652-11-S1-P247
Salem AH, Chiu YL, Valdes JM, Nilius AM, Klein CE (2015) A novel ritonavir paediatric powder formulation is bioequivalent to ritonavir oral solution with a similar food effect. Antivir Ther 20(4):425–432. https://doi.org/10.3851/imp2932
Mathias AA, West S, Hui J, Kearney BP (2009) Dose-response of ritonavir on hepatic CYP3A activity and elvitegravir oral exposure. Clin Pharmacol Ther 85(1):64–70. https://doi.org/10.1038/clpt.2008.168
Acknowledgements
Venetoclax (ABT-199/GDC-0199) is being developed in collaboration between AbbVie and Genentech. We thank the subjects who participated in this trial and their families; the study coordinators and the support staff at the clinical site; and AbbVie and Genentech venetoclax team members.
This study was supported by AbbVie in collaboration with Genentech/Roche. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, and analysis and interpretation of data as well as the writing, review, and approval of the manuscript. Medical writing support was provided by Therese Stickler, a freelance writer under contract with AbbVie, and Allison Kitten, an employee of AbbVie. Kevin J. Freise, Beibei Hu, and Ahmed Hamed Salem are employees of AbbVie and may hold AbbVie stock or stock options.
Funding
This study was supported by AbbVie in collaboration with Genentech/Roche. Venetoclax (ABT-199/GDC-0199) is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, and analysis and interpretation of data as well as the writing, review, and approval of the manuscript.
Author information
Authors and Affiliations
Contributions
KJF, BH, and AHS contributed to the study design, data analysis, interpreting of results, writing the manuscript, and approving the final version.
Corresponding author
Ethics declarations
Research involving human participants
The study was performed in accordance with the protocol, International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
Kevin J. Freise, Beibei Hu, and Ahmed Hamed Salem are employees of AbbVie and may hold AbbVie stock or stock options.
Rights and permissions
About this article
Cite this article
Freise, K.J., Hu, B. & Salem, A.H. Impact of ritonavir dose and schedule on CYP3A inhibition and venetoclax clinical pharmacokinetics. Eur J Clin Pharmacol 74, 413–421 (2018). https://doi.org/10.1007/s00228-017-2403-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2403-3