Abstract
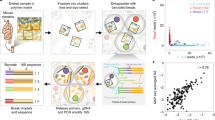
Metagenomic analysis of colonic mucosa-associated microbes has been complicated by technical challenges that disrupt or alter community structure and function. In the present study, we determined the feasibility of laser capture microdissection (LCM) of intact regional human colonic mucosa-associated microbes followed by phi29 multiple displacement amplification (MDA) and massively parallel sequencing for metagenomic analysis. Samples were obtained from the healthy human subject without bowel preparation and frozen sections immediately prepared. Regional mucosa-associated microbes were successfully dissected using LCM with minimal contamination by host cells, their DNA extracted and subjected to phi29 MDA with a high fidelity, prior to shotgun sequencing using the GS-FLX DNA sequencer. Metagenomic analysis of approximately 67 million base pairs of DNA sequences from two samples revealed that the metabolic functional profiles in mucosa-associated microbes were as diverse as those reported in feces, specifically the representation of functional genes associated with carbohydrate, protein, and nucleic acid utilization. In summary, these studies demonstrate the feasibility of the approach to study the structure and metagenomic profiles of human intestinal mucosa-associated microbial communities at small spatial scales.





Similar content being viewed by others
References
Abdo Z, Schuette UM, Bent SJ, Williams CJ, Forney LJ, Joyce P (2006) Statistical methods for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environ Microbiol 8:929–938
Atuma C, Strugala V, Allen A, Holm L (2001) The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280:G922–G929
Blatt R, Srinivasan S (2008) Defining disease with laser precision: laser capture microdissection in gastroenterology. Gastroenterology 135:364–369
Bollinger RR, Barbas AS, Bush EL, Lin SS, Parker W (2007) Biofilms in the normal human large bowel: fact rather than fiction. Gut 56:1481–1482
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2008) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145
Drlica K, Rouviere-Yaniv J (1987) Histonelike proteins of bacteria. Microbiol Rev 51:301–319
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science 308:1635–1638
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE (2006) Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1359
Green GL, Brostoff J, Hudspith B, Michael M, Mylonaki M, Rayment N, Staines N, Sanderson J, Rampton DS, Bruce KD (2006) Molecular characterization of the bacteria adherent to human colorectal mucosa. J Appl Microbiol 100:460–469
Klitgaard K, Molbak L, Jensen TK, Lindboe CF, Boye M (2005) Laser capture microdissection of bacterial cells targeted by fluorescence in situ hybridization. Biotechniques 39:864–868
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306
Lozupone C, Hamady M, Knight R (2006) UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform 7:371
Meyer F, Paarmann D, D’Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A, Wilkening J, Edwards RA (2008) The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinform 9:386
Michie MG (1982) Use of the Bray-Curtis similarity measure in cluster analysis of foraminiferal data. Math Geol 14:661–667
Molbak L, Klitgaard K, Jensen TK, Fossi M, Boye M (2006) Identification of a novel, invasive, not-yet-cultivated Treponema sp. in the large intestine of pigs by PCR amplification of the 16S rRNA gene. J Clin Microbiol 44:4537–4540
Pfister CA, Meyer F, Antonopoulos DA (2010) Metagenomic profiling of a microbial assemblage associated with the california mussel: a node in networks of carbon and nitrogen cycling. PLoS ONE 5:e10518
Pham VD, Konstantinidis KT, Palden T, DeLong EF (2008) Phylogenetic analyses of ribosomal DNA-containing bacterioplankton genome fragments from a 4000 m vertical profile in the North Pacific Subtropical Gyre. Environ Microbiol 10:2313–2330
Sakamoto M, Hayashi H, Benno Y (2003) Terminal restriction fragment length polymorphism analysis for human fecal microbiota and its application for analysis of complex bifidobacterial communities. Microbiol Immunol 47:133–142
Schmidt TM, Relman DA (1994) Phylogenetic identification of uncultured pathogens using ribosomal RNA sequences. Meth Enzymol 235:205–222
Simone NL, Bonner RF, Gillespie JW, Emmert-Buck MR, Liotta LA (1998) Laser-capture microdissection: opening the microscopic frontier to molecular analysis. Trends Genet 14:272–276
Sonnenburg JL, Angenent LT, Gordon JI (2004) Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol 5:569–573
Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H (2002) Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54
Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H (2005) Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43:3380–3389
Swinger KK, Rice PA (2004) IHF and HU: flexible architects of bent DNA. Curr Opin Struct Biol 14:28–35
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tringe SG, von Mering C, Kobayashi A, Salamov AA, Chen K, Chang HW, Podar M, Short JM, Mathur EJ, Detter JC, Bork P, Hugenholtz P, Rubin EM (2005) Comparative metagenomics of microbial communities. Science 308:554–557
Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC (2009) 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3:944–954
Zhang K, Martiny AC, Reppas NB, Barry KW, Malek J, Chisholm SW, Church GM (2006) Sequencing genomes from single cells by polymerase cloning. Nat Biotechnol 24:680–686
Acknowledgments
We thank Kevin White and the Institute for Genomics and Systems Biology at the University of Chicago for their support and technical assistance. We would like to acknowledge the following grant support: Digestive Disease Research Core Center (P30 DK42086; EBC), R21HG004858 (EBC), R01 HG004906 (VY), UH3 UH3DK083993 (VY), The Goldgraber fellowship foundation (LL), R01 5R01GM062344-11 (JA), and Research Fellowship Award (YW) from the Crohn’s and Colitis Foundation of America. We would also like to thank the Gastrointestinal Research Foundation of Chicago and Peter and Carol Goldman for supporting the microbiome research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yunwei Wang and Dionysios A. Antonopoulos have contributed equally to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Minimal contamination of host eukaryotic DNA by LCM technique. Host epithelial cell mixed in mucus layer is distinct by its red staining and separated first before microdissecting the mucosa-associated microbes (×400). Arrow shows one epithelial cell in the mucus layer (PDF 2123 kb)
Supplementary Fig. 2
Reproducibility of T-RFLP method. To investigate the reproducibility of T-RFLP method in this study, 16S rRNA gene was amplified from the same DNA template using three independent PCR reactions and digested by MspI. Digested PCR products were dialysed and sequenced. Variations within three profiles were evaluated. Fragment size in base pairs is shown at the top and relative peak heights are shown as relative fluorescence. GeneScan-500 internal size standard and 5′ terminal restriction fragments are shown as red and blue peaks, respectively. The level of methodological variability was analyzed by calculating peak position and peak area from the triplicate profiles. The variation from triplicate PCR reactions from the same DNA sample ranged from 1.7% to 3.2% which implied a high reproducibility of this method. (PDF 226 kb)
Supplementary Fig. 3
Phylogenetic relationships among the OTUs detected in LCM DNA samples before and after phi29 amplification. The representative 61 clone numbers are listed in the tree from right colon and 72 clone numbers are listed in the tree from left colon. Red branches were clones obtained from 16S rRNA gene library built up from un-amplified DNA and blue branches were clones from 16S rRNA gene library built up from phi29-amplified DNA. Sequences were aligned with RDP and are given the name at the Genus level. Bootstrap values are based on 100 replications (GIF 23 kb)
Supplementary Fig. 4
Relative distribution of classified sequences related to carbohydrate metabolism in human colon samples. Note the inverse representation of the majority of the sub-classes in the two samples (PDF 57 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Antonopoulos, D.A., Zhu, X. et al. Laser capture microdissection and metagenomic analysis of intact mucosa-associated microbial communities of human colon. Appl Microbiol Biotechnol 88, 1333–1342 (2010). https://doi.org/10.1007/s00253-010-2921-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2921-8