Abstract
Purpose
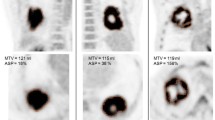
Asphericity (ASP) is a tumour shape descriptor based on the PET image. It quantitates the deviation from spherical of the shape of the metabolic tumour volume (MTV). In order to identify its biological correlates, we investigated the relationship between ASP and clinically relevant histopathological and molecular signatures in non-small-cell lung cancer (NSCLC).
Methods
The study included 83 consecutive patients (18 women, aged 66.4 ± 8.9 years) with newly diagnosed NSCLC in whom PET/CT with 18F-FDG had been performed prior to therapy. Primary tumour resection specimens and core biopsies were used for basic histopathology and determination of the Ki-67 proliferation index. EGFR status, VEGF, p53 and ALK expression were obtained in a subgroup of 44 patients. The FDG PET images of the primary tumours were delineated using an automatic algorithm based on adaptive thresholding taking into account local background. In addition to ASP, SUVmax, MTV and some further descriptors of shape and intratumour heterogeneity were assessed as semiquantitative PET measures.
Results
SUVmax, MTV and ASP were associated with pathological T stage (Kruskal-Wallis, p = 0.001, p < 0.0005 and p < 0.0005, respectively) and N stage (p = 0.017, p = 0.003 and p = 0.002, respectively). Only ASP was associated with M stage (p = 0.026). SUVmax, MTV and ASP were correlated with Ki-67 index (Spearman’s rho = 0.326/p = 0.003, rho = 0.302/p = 0.006 and rho = 0.271/p = 0.015, respectively). The latter correlations were considerably stronger in adenocarcinomas than in squamous cell carcinomas. ASP, but not SUVmax or MTV, showed a tendency for a significant association with the extent of VEGF expression (p = 0.058). In multivariate Cox regression analysis, ASP (p < 0.0005) and the presence of distant metastases (p = 0.023) were significantly associated with progression-free survival. ASP (p = 0.006), the presence of distant metastases (p = 0.010), and Ki-67 index (p = 0.062) were significantly associated with overall survival.
Conclusion
The ASP of primary NSCLCs on FDG PET images is associated with tumour dimensions and molecular markers of proliferation and angiogenesis.






Similar content being viewed by others
References
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7.
Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–38. doi:10.1177/1758834011401951.
Roy S, Pathy S, Kumar R, Mohanti BK, Raina V, Jaiswal A, et al. Efficacy of 18F-fluorodeoxyglucose positron emission tomography/computed tomography as a predictor of response in locally advanced non-small-cell carcinoma of the lung. Nucl Med Commun. 2016;37:129–38. doi:10.1097/MNM.0000000000000422.
Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54:19–26. doi:10.2967/jnumed.112.107375.
Eary JF, O’Sullivan F, O’Sullivan J, Conrad EU. Spatial heterogeneity in sarcoma 18F-FDG uptake as a predictor of patient outcome. J Nucl Med. 2008;49:1973–9. doi:10.2967/jnumed.108.053397.
El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009;42:1162–71. doi:10.1016/j.patcog.2008.08.011.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369–78. doi:10.2967/jnumed.110.082404.
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi:10.1038/ncomms5006.
Apostolova I, Rogasch J, Buchert R, Wertzel H, Achenbach HJ, Schreiber J, et al. Quantitative assessment of the asphericity of pretherapeutic FDG uptake as an independent predictor of outcome in NSCLC. BMC Cancer. 2014;14:896. doi:10.1186/1471-2407-14-896.
Apostolova I, Steffen IG, Wedel F, Lougovski A, Marnitz S, Derlin T, et al. Asphericity of pretherapeutic tumour FDG uptake provides independent prognostic value in head-and-neck cancer. Eur Radiol. 2014;24:2077–87. doi:10.1007/s00330-014-3269-8.
Hofheinz F, Lougovski A, Zophel K, Hentschel M, Steffen IG, Apostolova I, et al. Increased evidence for the prognostic value of primary tumor asphericity in pretherapeutic FDG PET for risk stratification in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2015;42:429–37. doi:10.1007/s00259-014-2953-x.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40:133–40. doi:10.1007/s00259-012-2247-0.
Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi:10.1056/NEJMra0802714.
Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol. 2011;2011:583929. doi:10.1155/2011/583929.
Herbst RS, Bunn Jr PA. Targeting the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res. 2003;9:5813–24.
Nana-Sinkam SP, Powell CA. Molecular biology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e30S–9S. doi:10.1378/chest.12-2346.
Hofheinz F, Potzsch C, Oehme L, Beuthien-Baumann B, Steinbach J, Kotzerke J, et al. Automatic volume delineation in oncological PET. Evaluation of a dedicated software tool and comparison with manual delineation in clinical data sets. Nuklearmedizin. 2012;51:9–16. doi:10.3413/Nukmed-0419-11-07.
Hofheinz F, Langner J, Petr J, Beuthien-Baumann B, Steinbach J, Kotzerke J, et al. An automatic method for accurate volume delineation of heterogeneous tumors in PET. Med Phys. 2013;40:082503. doi:10.1118/1.4812892.
Barber CB, Dobkin DP, Huhdanpaa H. The Quickhull algorithm for convex hulls. ACM Trans Math Softw. 1996;22:469–83.
Hatt M, Majdoub M, Vallieres M, Tixier F, Le Rest CC, Groheux D, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med. 2015;56:38–44. doi:10.2967/jnumed.114.144055.
Vallieres M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60:5471–96. doi:10.1088/0031-9155/60/14/5471.
van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging. 2011;38:1636–47.
Shin Y, Han S, Chung E, Chung S. Intratumoral phenotypic heterogeneity as an encourager of cancer invasion. Integr Biol (Camb). 2014;6:654–61.
Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–60. doi:10.1097/PAS.0b013e31821a0696.
Groheux D, Majdoub M, Tixier F, Le Rest CC, Martineau A, Merlet P, et al. Do clinical, histological or immunohistochemical primary tumour characteristics translate into different (18)F-FDG PET/CT volumetric and heterogeneity features in stage II/III breast cancer? Eur J Nucl Med Mol Imaging. 2015;42:1682–91. doi:10.1007/s00259-015-3110-x.
Schuurbiers OC, Meijer TW, Kaanders JH, Looijen-Salamon MG, de Geus-Oei LF, van der Drift MA, et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J Thorac Oncol. 2014;9:1485–93. doi:10.1097/JTO.0000000000000286.
Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Vigil Diaz C, Lopez-Picazo JM, et al. Role of [18F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:2058–65. doi:10.1007/s00259-014-2833-4.
Del Gobbo A, Pellegrinelli A, Gaudioso G, Castellani M, Zito Marino F, Franco R, et al. Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology. 2016;68:746–51. doi:10.1111/his.12808.
Tixier F, Hatt M, Valla C, Fleury V, Lamour C, Ezzouhri S, et al. Visual versus quantitative assessment of intratumor 18F-FDG PET uptake heterogeneity: prognostic value in non-small cell lung cancer. J Nucl Med. 2014;55:1235–41. doi:10.2967/jnumed.113.133389.
Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, Eder V, et al. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS One. 2014;9, e94017. doi:10.1371/journal.pone.0094017.
Warth A, Cortis J, Soltermann A, Meister M, Budczies J, Stenzinger A, et al. Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer. 2014;111:1222–9. doi:10.1038/bjc.2014.402.
da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi:10.1146/annurev-pathol-011110-130206.
Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1alpha in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia. 2015;17:190–200. doi:10.1016/j.neo.2014.12.008.
Ranayhossaini DJ, Lu J, Mabus J, Gervais A, Lingham RB, Fursov N. EGF potentiation of VEGF production is cell density dependent in H292 EGFR wild type NSCLC cell line. Int J Mol Sci. 2014;15:17686–704. doi:10.3390/ijms151017686.
Kaida H, Kawahara A, Hayakawa M, Hattori S, Kurata S, Fujimoto K, et al. The difference in relationship between 18F-FDG uptake and clinicopathological factors on thyroid, esophageal, and lung cancers. Nucl Med Commun. 2014;35:36–43. doi:10.1097/MNM.0000000000000019.
Sauter AW, Winterstein S, Spira D, Hetzel J, Schulze M, Mueller M, et al. Multifunctional profiling of non-small cell lung cancer using 18F-FDG PET/CT and volume perfusion CT. J Nucl Med. 2012;53:521–9. doi:10.2967/jnumed.111.097865.
Kaira K, Oriuchi N, Shimizu K, Ishikita T, Higuchi T, Imai H, et al. Correlation of angiogenesis with 18F-FMT and 18F-FDG uptake in non-small cell lung cancer. Cancer Sci. 2009;100:753–8. doi:10.1111/j.1349-7006.2008.01077.x.
Makinoshima H, Takita M, Matsumoto S, Yagishita A, Owada S, Esumi H, et al. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J Biol Chem. 2014;289:20813–23. doi:10.1074/jbc.M114.575464.
De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, et al. Reversal of Warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21:5110–20. doi:10.1158/1078-0432.CCR-15-0375.
Jakobsen JN, Sorensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer. 2013;79:1–7. doi:10.1016/j.lungcan.2012.10.008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
None.
Ethical approval
All procedures performed in this study were approved by the Institutional Ethics Committee (reference number, 159/13; RAD233) and were in accordance with ethical standards and with the principles of the 1964 Declaration of Helsinki and its later amendments. This article does not describe any studies with animals performed by any of the authors.
Informed consent
Informed consent for retrospective data evaluation was obtained from all participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Apostolova, I., Ego, K., Steffen, I.G. et al. The asphericity of the metabolic tumour volume in NSCLC: correlation with histopathology and molecular markers. Eur J Nucl Med Mol Imaging 43, 2360–2373 (2016). https://doi.org/10.1007/s00259-016-3452-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3452-z