Abstract
Purposes
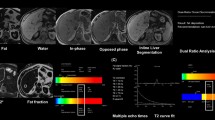
To evaluate the feasibility of simultaneous quantification of liver iron concentration (LIC) and fat fraction (FF) using water-fat separation and quantitative susceptibility mapping (QSM).
Methods
Forty-five patients suspected of liver iron overload (LIO) were included. A volumetric interpolated breath-hold examination sequence for QSM and FF, a fat-saturated gradient echo sequence for R2*, a spin echo sequence for LIC measurements and MRS analyses for FF (FF-MRS) were performed. Magnetic susceptibility and FF were calculated using a water-fat separation method (FF-MRI). Correlation and receiver operating characteristic analyses were performed.
Results
Magnetic susceptibility showed strong correlation with LIC (rs=0.918). The optimal susceptibility cut-off values were 0.34, 0.63, 1.29 and 2.23 ppm corresponding to LIC thresholds of 1.8, 3.2, 7.0 and 15.0 mg/g dry weight. The area under the curve (AUC) were 0.948, 0.970, 1 and 1, respectively. No difference in AUC was found between susceptibility and R2* at all LIC thresholds. Correlation was found between FF-MRI and FF-MRS (R2=0.910).
Conclusions
QSM has a high diagnostic performance for LIC quantification, similar to that of R2*. FF-MRI provides simultaneous fat quantification. Findings suggest QSM in combination with water-fat separation has potential value for evaluating LIO, especially in cases with coexisting steatosis.
Key Points
• Magnetic susceptibility showed strong correlation with LIC (r s =0.918).
• QSM showed high diagnostic performance for LIC, similar to that of R 2 * .
• Simultaneously estimated FF-MRI showed strong correlation with MR-Spectroscopy-based FF (R 2 =0.910).
• QSM combining water-fat separation has quantitative value for LIO with coexisted steatosis.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- FF-MRI:
-
FF calculated with a water-fat separation method
- FF-MRS:
-
MR Spectroscopy based FF
- FF:
-
Fat fraction
- FOV:
-
Field of view
- GRE:
-
Gradient echo
- ICC:
-
Intraclass correlation coefficients
- LIC:
-
Liver iron concentration
- LIO:
-
Liver iron overload
- mg/g dw:
-
mg/g dry weight
- QSM:
-
Quantitative susceptibility mapping
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- VIBE:
-
Volumetric interpolated breath-hold examination
References
Brittenham GM, Badman DG, National Institute of D, Digestive, Kidney Diseases W (2003) Noninvasive measurement of iron: report of an NIDDK workshop. Blood 101:15–19
Vermylen C (2008) What is new in iron overload? Eur J Pediatr 167:377–381
Chou ST, Fasano RM (2016) Management of Patients with Sickle Cell Disease Using Transfusion Therapy: Guidelines and Complications. Hematol Oncol Clin North Am 30:591–608
Wood JC (2015) Estimating tissue iron burden: current status and future prospects. Br J Haematol 170:15–28
Wood JC, Enriquez C, Ghugre N et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106:1460–1465
Hankins JS, McCarville MB, Loeffler RB et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113:4853–4855
Kuhn JP, Hernando D, Munoz del Rio A et al (2012) Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology 265:133–142
Moirand R, Mortaji AM, Loreal O, Paillard F, Brissot P, Deugnier Y (1997) A new syndrome of liver iron overload with normal transferrin saturation. Lancet 349:95–97
George DK, Goldwurm S, MacDonald GA et al (1998) Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology 114:311–318
Wood MJ, Powell LW, Dixon JL, Ramm GA (2012) Clinical cofactors and hepatic fibrosis in hereditary hemochromatosis: the role of diabetes mellitus. Hepatology 56:904–911
Kuhn JP, Meffert P, Heske C et al (2017) Prevalence of Fatty Liver Disease and Hepatic Iron Overload in a Northeastern German Population by Using Quantitative MR Imaging. Radiology 284:706–716
Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18:337–357 ix
Henninger B, Zoller H, Rauch S et al (2015) R2* relaxometry for the quantification of hepatic iron overload: biopsy-based calibration and comparison with the literature. Rofo 187:472–479
Sanches-Rocha L, Serpa B, Figueiredo E, Hamerschlak N, Baroni R (2013) Comparison between multi-echo T2* with and without fat saturation pulse for quantification of liver iron overload. Magn Reson Imaging 31:1704–1708
Krafft AJ, Loeffler RB, Song R et al (2016) Does fat suppression via chemically selective saturation affect R2*-MRI for transfusional iron overload assessment? A clinical evaluation at 1.5T and 3T. Magn Reson Med 76:591–601
Meloni A, Tyszka JM, Pepe A, Wood JC (2015) Effect of inversion recovery fat suppression on hepatic R2* quantitation in transfusional siderosis. AJR Am J Roentgenol 204:625–629
Powell EE, Ali A, Clouston AD et al (2005) Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology 129:1937–1943
Galimberti S, Trombini P, Bernasconi DP et al (2015) Simultaneous liver iron and fat measures by magnetic resonance imaging in patients with hyperferritinemia. Scand J Gastroenterol 50:429–438
Franca M, Alberich-Bayarri A, Marti-Bonmati L et al (2017) Accurate simultaneous quantification of liver steatosis and iron overload in diffuse liver diseases with MRI. Abdom Radiol (NY) 42:1434–1443
Henninger B, Zoller H, Kannengiesser S, Zhong X, Jaschke W, Kremser C (2017) 3D Multiecho Dixon for the Evaluation of Hepatic Iron and Fat in a Clinical Setting. J Magn Reson Imaging 46:793–800
Taylor BA, Loeffler RB, Song R, McCarville MB, Hankins JS, Hillenbrand CM (2012) Simultaneous field and R2 mapping to quantify liver iron content using autoregressive moving average modeling. J Magn Reson Imaging 35:1125–1132
Liu C, Wei H, Gong NJ, Cronin M, Dibb R, Decker K (2015) Quantitative susceptibility mapping: contrast mechanisms and clinical applications. Tomography 1:3–17
Wei H, Xie L, Dibb R et al (2016) Imaging whole-brain cytoarchitecture of mouse with MRI-based quantitative susceptibility mapping. Neuroimage 137:107–115
Sharma SD, Fischer R, Schoennagel BP et al (2017) MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: Comparison with SQUID-based biomagnetic liver susceptometry. Magn Reson Med 78:264–270
Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB (2008) Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 60:1122–1134
Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR (2009) Measurement of hepatic lipid: high-speed T2-corrected multiecho acquisition at 1H MR spectroscopy--a rapid and accurate technique. Radiology 252:568–576
Hamilton G, Yokoo T, Bydder M et al (2011) In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed 24:784–790
Wu B, Li W, Guidon A, Liu C (2012) Whole brain susceptibility mapping using compressed sensing. Magn Reson Med 67:137–147
Wei H, Dibb R, Zhou Y et al (2015) Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed 28:1294–1303
Wei H, Zhang Y, Gibbs E, Chen NK, Wang N, Liu C (2016) Joint 2D and 3D phase processing for quantitative susceptibility mapping: application to 2D echo-planar imaging. NMR Biomed. https://doi.org/10.1002/nbm.3501
Feng Y, He T, Gatehouse PD et al (2013) Improved MRI R2 * relaxometry of iron-loaded liver with noise correction. Magn Reson Med 70:1765–1774
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Sharma SD, Hernando D, Horng DE, Reeder SB (2015) Quantitative susceptibility mapping in the abdomen as an imaging biomarker of hepatic iron overload. Magn Reson Med 74:673–683
Longo R, Ricci C, Masutti F et al (1993) Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol 28:297–302
Wood JC (2014) Guidelines for quantifying iron overload. Hematology Am Soc Hematol Educ Program 2014:210–215
Olivieri NF, Brittenham GM (1997) Iron-chelating therapy and the treatment of thalassemia. Blood 89:739–761
Harrison SA, Bacon BR (2005) Relation of hemochromatosis with hepatocellular carcinoma: epidemiology, natural history, pathophysiology, screening, treatment, and prevention. Med Clin North Am 89:391–409
Kang BK, Yu ES, Lee SS et al (2012) Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol 47:368–375
Hong CW, Mamidipalli A, Hooker JC et al (2017) MRI proton density fat fraction is robust across the biologically plausible range of triglyceride spectra in adults with nonalcoholic steatohepatitis. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25845
Satkunasingham J, Besa C, Bane O et al (2015) Liver fat quantification: Comparison of dual-echo and triple-echo chemical shift MRI to MR spectroscopy. Eur J Radiol 84:1452–1458
Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR (2014) Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med 72:1353–1365
Yokoo T, Browning JD (2014) Fat and iron quantification in the liver: past, present, and future. Top Magn Reson Imaging 23:73–94
St Pierre TG, El-Beshlawy A, Elalfy M et al (2014) Multicenter validation of spin-density projection-assisted R2-MRI for the noninvasive measurement of liver iron concentration. Magn Reson Med 71:2215–2223
Xie L, Sparks MA, Li W et al (2013) Quantitative susceptibility mapping of kidney inflammation and fibrosis in type 1 angiotensin receptor-deficient mice. NMR Biomed 26:1853–1863
Krafft AJ, Loeffler RB, Song R et al (2017) Quantitative ultrashort echo time imaging for assessment of massive iron overload at 1.5 and 3 Tesla. Magn Reson Med. https://doi.org/10.1002/mrm.26592
Luo XF, Xie XQ, Cheng S et al (2015) Dual-energy CT for patients suspected of having liver iron overload: can virtual iron content imaging accurately quantify liver iron content? Radiology 277:95–103
Wang Y, Liu T (2015) Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med 73:82–101
Henninger B, Kremser C, Rauch S et al (2012) Evaluation of MR imaging with T1 and T2* mapping for the determination of hepatic iron overload. Eur Radiol 22:2478–2486
Acknowledgements
We thank Stephan Kannengiesser for helpful discussion.
Funding
This study has received funding by National Natural Science Foundation of China (81671649), National Institute of Mental Health (R01MH096979, R24MH106096), National Institute of Neurological Disorders and Stroke (R01NS079653), and National Heart, Lung, and Blood Institute (R21HL122759).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Fuhua Yan.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Caixia Fu and Xu Yan are employees of Siemens Healthcare.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lin, H., Wei, H., He, N. et al. Quantitative susceptibility mapping in combination with water-fat separation for simultaneous liver iron and fat fraction quantification. Eur Radiol 28, 3494–3504 (2018). https://doi.org/10.1007/s00330-017-5263-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5263-4