Abstract
Objective
To evaluate Gd-EOB-DTPA-enhanced MRI for quantitative assessment of liver organ damage after hepatic ischaemia reperfusion injury (IRI) in mice.
Methods
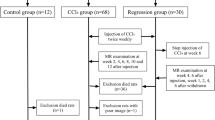
Partial hepatic IRI was induced in C57Bl/6 mice (n = 31) for 35, 45, 60 and 90 min. Gd-EOB-DTPA-enhanced MRI was performed 1 day after surgery using a 3D-FLASH sequence. A subgroup of n = 9 animals with 60 min IRI underwent follow-up with MRI and histology 7 days after IRI. The total liver volume was determined by manual segmentation of the entire liver. The volume of functional, contrast-enhanced liver parenchyma was quantified by a region growing algorithm (visual threshold) and an automated segmentation (Otsu’s method). The percentages of functional, contrast-enhanced and damaged non-enhanced parenchyma were calculated according to these volumes. MRI data was correlated with serum liver enzyme concentrations and histologically quantified organ damage using periodic acid–Schiff (PAS) staining.
Results
The percentage of functional (contrasted) liver parenchyma decreased significantly with increasing ischaemia times (control, 94.4 ± 3.3%; 35 min IRI, 89.3 ± 4.1%; 45 min IRI, 87.9 ± 3.3%; 60 min IRI, 68 ± 10.5%, p < 0.001 vs. control; 90 min IRI, 55.9 ± 11.5%, p < 0.001 vs. control). The percentage of non-contrasted liver parenchyma correlated with histologically quantified liver organ damage (r = 0.637, p < 0.01) and serum liver enzyme elevations (AST r = 0.577, p < 0.01; ALT r = 0.536, p < 0.05). Follow-up MRI visualized recovery of functional liver parenchyma (71.5 ± 8.7% vs. 84 ± 2.1%, p < 0.05), consistent with less histological organ damage on day 7.
Conclusion
We demonstrated the feasibility of Gd-EOB-DTPA-enhanced MRI for non-invasive quantification of damaged liver parenchyma following IRI in mice. This novel methodology may refine the characterization of liver disease and could have application in future studies targeting liver organ damage.
Key Points
• Prolonged ischaemia times in partial liver IRI increase liver organ damage.
• Gd-EOB-DTPA-enhanced MRI at hepatobiliary phase identifies damaged liver volume after hepatic IRI.
• Damaged liver parenchyma quantified with MRI correlates with histological liver damage.
• Hepatobiliary phase Gd-EOB-DTPA-enhanced MRI enables non-invasive assessment of recovery from liver injury.





Similar content being viewed by others

Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ATP:
-
Adenosine triphosphate
- B6:
-
C57Bl/6JHan-ztm
- CT:
-
Computed tomography
- FLASH:
-
3D-fast low angle shot
- Gd-EOB-DTPA:
-
Gadolinium ethoxybenzyl Diethylenetriaminepentaacetic acid
- IRI:
-
Ischaemia reperfusion injury
- MRI:
-
Magnetic resonance imaging
- MRP2:
-
Multidrug resistance-associated protein 2
- OATP1:
-
Organic anion-transporting polypeptide 1
- PAS:
-
Periodic acid Schiff
- SD:
-
Standard deviation
References
Kulik U, Schrem H, Bektas H et al (2016) Prognostic relevance of hematological profile before resection for colorectal liver metastases. J Surg Res 206:498–506
Kulik U, Bektas H, Klempnauer J, Lehner F (2013) Repeat liver resection for colorectal metastases. Br J Surg 100:926–932
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190:255–266
Kelly DM, Shiba H, Nakagawa S et al (2011) Hepatic blood flow plays an important role in ischaemia-reperfusion injury. Liver Transpl 17:1448–1456
Glanemann M, Langrehr JM, Stange BJ et al (2003) Clinical implications of hepatic preservation injury after adult liver transplantation. Am J Transplant 3:1003–1009
Rosen HR, Martin P, Goss J et al (1998) Significance of early aminotransferase elevation after liver transplantation. Transplantation 65:68–72
Jaeschke H, Woolbright B (2013) Injury by targeting reactive oxygen species. Transpl Rev 26:103–114
Van Beers BE, Pastor CM, Hussain HK (2012) Primovist, eovist: what to expect? J Hepatol 57:421–429
Jia J, Puls D, Oswald S et al (2014) Characterization of the intestinal and hepatic uptake/efflux transport of the magnetic resonance imaging contrast agent gadolinium-ethoxylbenzyl-diethylenetriamine-pentaacetic acid. Invest Radiol 49:78–86
Jeong WK, Kim YK, Song KD et al (2013) The MR imaging diagnosis of liver diseases using gadoxetic acid: emphasis on hepatobiliary phase. Clin Mol Hepatol 19:360–366
Kukuk GM, Schaefer SG, Fimmers R et al (2014) Hepatobiliary magnetic resonance imaging in patients with liver disease: correlation of liver enhancement with biochemical liver function tests. Eur Radiol 24:2482–2490
Verloh N, Haimerl M, Zeman F et al (2014) Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol 24:1013–1019
Byk K, Jasinski K, Bartel Z et al (2016) MRI-based assessment of liver perfusion and hepatocyte injury in the murine model of acute hepatitis. MAGMA. https://doi.org/10.1007/s10334-016-0563-2
Yamada T, Obata A, Kashiwagi Y et al (2016) Gd-EOB-DTPA-enhanced-MR imaging in the inflammation stage of nonalcoholic steatohepatitis (NASH) in mice. Magn Reson Imaging 34:724–729
Yamashita T, Kitao A, Matsui O et al (2014) Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology 60:1674–1685
Uchida Y, Freitas MCS, Zhao D et al (2009) The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischaemia and reperfusion. Liver Transplant 15:939–947
Messiha BAS, Abo-Youssef AM (2015) Protective effects of fish oil, allopurinol, and verapamil on hepatic ischaemia-reperfusion injury in rats. J Nat Sci Biol Med 6:351–355
Korkusuz H, Knau LL, Kromen W et al (2012) Different signal intensity at Gd-EOB-DTPA compared with Gd-DTPA-enhanced MRI in hepatocellular carcinoma transgenic mouse model in delayed phase hepatobiliary imaging. J Magn Reson Imaging 35:1397–1402
Kiryu S, Inoue Y, Watanabe M et al (2009) Evaluation of gadoxetate disodium as a contrast agent for mouse liver imaging: comparison with gadobenate dimeglumine. J Magn Reson Imaging 27:101–107
Portnoy S, Bishop J, Dazai J et al (2008) Characterization of signal ehnacement following the intraperitoneal injection of gadolinium based contrast agents. Proc 16th Sci Meet Int Soc Magn Reson Med Toronto 54:3206
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern B Cybern 9:62–66
Hui H, Ma W, Cui J et al (2017) Periodic acid-Schiff staining method for function detection of liver cells is affected by 2% horse serum in induction medium. Mol Med Rep 16:8062–8068
Chouker A, Lizak M, Schimel D et al (2008) Comparison of Fenestra VC contrast-enhanced computed tomography imaging with gadopentetate dimeglumine and ferucarbotran magnetic resonance imaging for the in vivo evaluation of murine liver damage after ischaemia and reperfusion. Invest Radiol 43:77–91
Lu Y, Liu P, Fu P et al (2017) Comparison of the DWI and Gd-EOB-DTPA-enhanced MRI on assessing the hepatic ischaemia and reperfusion injury after partial hepatectomy. Biomed Pharmacother 86:118–126
Konishi T, Lentsch AB (2017) Hepatic ischaemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr 17:277–287
Li H, Lu J, Zhou X et al (2017) Quantitative analysis of hepatic microcirculation in rabbits after liver ischaemia-reperfusion injury using contrast-enhanced ultrasound. Ultrasound Med Biol 43:2469–2476
Donner MG, Cebula P, Krienen A et al (2013) HbG200-mediated preinduction of heme oxygenase-1 improves bile flow and ameliorates pericentral downregulation of Bsep and MRP2 following experimental liver ischaemia and reperfusion. Biol Chem 394:97–112
Thorling CA, Roberts MS, Liu X et al (2014) Effects of long-term hepatic ischaemia-reperfusion injury on the function of p-glycoprotein in vivo in rats. J Pharm Pharm Sci 17:121–135
Thorling CA, Liu X, Burczynski FJ et al (2013) Intravital multiphoton microscopy can model uptake and excretion of fluorescein in hepatic ischaemia-reperfusion injury. J Biomed Opt 18:101306
Ban D, Kudo A, Sui S et al (2009) Decreased MRP2-dependent bile flow in the post-warm ischemic rat liver. J Surg Res 153:310–316
Tanaka Y, Chen C, Maher JM, Klaassen CD (2006) Kupffer cell-mediated downregulation of hepatic transporter expression in rat hepatic ischaemia-reperfusion. Transplantation 82:258–266
Schaub JR, Malato Y, Gormond C, Willenbring H (2014) Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep 8:933–939
Ogoke O, Oluwole J, Parashurama N (2017) Bioengineering considerations in liver regenerative medicine. J Biol Eng 11:46
Michalopoulos GK (2013) Principles of liver regeneration and growth homeostasis. Compr Physiol 3:485–513
Lafaro K, Buettner S, Maqsood H et al (2015) Defining post hepatectomy liver insufficiency: where do we stand? J Gastrointest Surg 19:2079–2092
Rahbari NN, Garden OJ, Padbury R et al (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149:713–724
Hyder O, Pulitano C, Firoozmand A et al (2013) A risk model to predict 90-day mortality among patients undergoing hepatic resection. J Am Coll Surg 216:1049–1056
Shimizu J, Dono K, Gotoh M et al (1999) Evaluation of regional liver function by gadolinium-EOB-DTPA-enhanced MR imaging. Dig Dis Sci 44:1330–1337
Ryeom H-K, Kim S-H, Kim J-Y et al (2004) Quantitative evaluation of liver function with MRI using Gd-EOB-DTPA. Korean J Radiol 5:231–239
Selzner N, Rudiger H, Graf R, Clavien P-A (2003) Protective strategies against ischemic injury of the liver. Gastroenterology 125:917–936
Teoh NC, Farrell GC (2003) Hepatic ischaemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 18:891–902
Acknowledgements
We thank Christian Bergen, Herle Chlebusch and Las Kaehler for their excellent technical support.
Funding
This study has received funding by Hannover Medical School (Junge Akademie Program) as well as REBIRTH Cluster of Excellence of Hannover Medical School.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Katja Hueper.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because of the nature of the study, which was experimental. No patient data is included.
Ethical approval
Approval from the institutional animal care committee was obtained.
Methodology
• experimental
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 113 kb)
Rights and permissions
About this article
Cite this article
Getzin, T., Gueler, F., Hartleben, B. et al. Gd-EOB-DTPA-enhanced MRI for quantitative assessment of liver organ damage after partial hepatic ischaemia reperfusion injury: correlation with histology and serum biomarkers of liver cell injury. Eur Radiol 28, 4455–4464 (2018). https://doi.org/10.1007/s00330-018-5380-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5380-8