Abstract
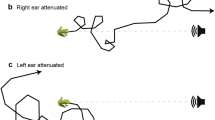
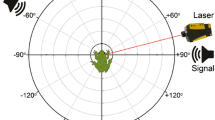
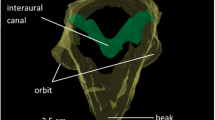
Anuran ears function as pressure difference receivers, and the amplitude and phase of tympanum vibrations are inherently directional, varying with sound incident angle. We quantified the nature of this directionality for Cope’s gray treefrog, Hyla chrysoscelis. We presented subjects with pure tones, advertisement calls, and frequency-modulated sweeps to examine the influence of frequency, signal level, lung inflation, and sex on ear directionality. Interaural differences in the amplitude of tympanum vibrations were 1–4 dB greater than sound pressure differences adjacent to the two tympana, while interaural differences in the phase of tympanum vibration were similar to or smaller than those in sound phase. Directionality in the amplitude and phase of tympanum vibration were highly dependent on sound frequency, and directionality in amplitude varied slightly with signal level. Directionality in the amplitude and phase of tone- and call-evoked responses did not differ between sexes. Lung inflation strongly affected tympanum directionality over a narrow frequency range that, in females, included call frequencies. This study provides a foundation for further work on the biomechanics and neural mechanisms of spatial hearing in H. chrysoscelis, and lends valuable perspective to behavioral studies on the use of spatial information by this species and other frogs.








Similar content being viewed by others
Abbreviations
- IAD:
-
Interaural amplitude difference
- ILD:
-
Interaural level difference
- IPD:
-
Interaural phase difference
- ITD:
-
Interaural time difference
- IVAD:
-
Interaural vibration amplitude difference
- IVPD:
-
Interaural vibration phase difference
- IVTD:
-
Interaural vibration timing difference
- TVA:
-
Tympanum vibration amplitude
- TVP:
-
Tympanum vibration phase
References
Aertsen AMHJ, Vlaming MSMG, Eggermont JJ, Johannesma PIM (1986) Directional hearing in the grassfrog (Rana temporaria L.). II. Acoustics and modelling of the auditory periphery. Hear Res 21(1):17–40. doi:10.1016/0378-5955(86)90043-2
Baugh AT, Ryan MJ (2010) Mate choice in response to dynamic presentation of male advertisement signals in tungara frogs. Anim Behav 79(1):145–152. doi:10.1016/j.anbehav.2009.10.015
Bee MA (2007a) Selective phonotaxis by male wood frogs (Rana sylvatica) to the sound of a chorus. Behav Ecol Sociobiol 61:955–966
Bee MA (2007b) Sound source segregation in grey treefrogs: spatial release from masking by the sound of a chorus. Anim Behav 74:549–558
Bee MA (2008) Finding a mate at a cocktail party: spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim Behav 75:1781–1791
Bee MA (2010) Spectral preferences and the role of spatial coherence in simultaneous integration in gray treefrogs (Hyla chrysoscelis). J Comp Psychol 124:412–424
Bee MA, Riemersma KK (2008) Does common spatial origin promote the auditory grouping of temporally separated signal elements in grey treefrogs? Anim Behav 76:831–843
Caldwell MS, Bee MA (2014) Spatial hearing in Cope’s gray treefrog: I. Open and closed loop experiments on sound localization in the presence and absence of noise. J Comp Physiol A. doi:10.1007/s00359-014-0882-6
Christensen-Dalsgaard J (2005) Directional hearing in nonmammalian tetrapods. In: Popper AN, Fay RR (eds) Sound source localization. Springer handbook of auditory research, vol 25. Springer, New York, pp 67–123
Christensen-Dalsgaard J (2011) Vertebrate pressure-gradient receivers. Hear Res 273(1–2):37–45. doi:10.1016/j.heares.2010.08.007
Christie K, Schul J, Feng AS (2010) Phonotaxis to male’s calls embedded within a chorus by female gray treefrogs, Hyla versicolor. J Comp Physiol A 196(8):569–579. doi:10.1007/s00359-010-0544-2
Ehret G, Tautz J, Schmitz B, Narins PM (1990) Hearing through the lungs: lung-eardrum transmission of sound in the frog Eleutherodactylus coqui. Naturwissenschaften 77(4):192–194
Ehret G, Keilwerth E, Kamada T (1994) The lung-eardrum pathway in three treefrog and four dendrobatid frog species: some properties of sound transmission. J Exp Biol 195:329–343
Farris HE, Ryan MJ (2011) Relative comparisons of call parameters enable auditory grouping in frogs. Nat Commun 2:410
Farris HE, Rand AS, Ryan MJ (2002) The effects of spatially separated call components on phonotaxis in túngara frogs: evidence for auditory grouping. Brain Behav Evol 60(3):181–188
Farris HE, Rand AS, Ryan MJ (2005) The effects of time, space and spectrum on auditory grouping in túngara frogs. J Comp Physiol A 191(12):1173–1183
Feng AS (1980) Directional characteristics of the acoustic receiver of the leopard frog (Rana pipiens): a study of 8th nerve auditory responses. J Acoust Soc Am 68(4):1107–1114
Feng AS, Capranica RR (1978) Sound localization in anurans II. Binaural interaction in superior olivary nucleus of the green tree frog (Hyla cinerea). J Neurophysiol 41(1):43–54
Feng AS, Shofner WP (1981) Peripheral basis of sound localization in anurans: acoustic properties of the frog’s ear. Hear Res 5(2–3):201–216
Feng AS, Gerhardt HC, Capranica RR (1976) Sound localization behavior of the green treefrog (Hyla cinerea) and the barking treefrog (Hyla gratiosa). J Comp Physiol A 107(3):241–252
Gerhardt HC (1975) Sound pressure levels and radiation patterns of vocalizations of some North American frogs and toads. J Comp Physiol A 102(1):1–12
Gerhardt HC (1995) Phonotaxis in female frogs and toads: execution and design of experiments. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC (eds) Methods in comparative psychoacoustics, vol 28. Birkhäuser Verlag, Basel, pp 209–220
Gerhardt HC, Bee MA (2007) Recognition and localization of acoustic signals. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians, vol 28. Springer handbook of auditory researchSpringer, New York, pp 113–146
Gerhardt HC, Huber F (eds) (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago University Press, Chicago
Gerhardt HC, Klump GM (1988) Phonotactic responses and selectivity of barking treefrogs (Hyla gratiosa) to chorus sounds. J Comp Physiol A 163(6):795–802
Gerhardt HC, Rheinlaender J (1980) Accuracy of sound localization in a miniature dendrobatid frog. Naturwissenschaften 67(7):362–363
Gerhardt HC, Rheinlaender J (1982) Localization of an elevated sound source by the green tree frog. Science 217(4560):663–664
Greenhouse SW, Geisser S (1959) On methods in the analysis of profile data. Psychometrika 24(2):95–112
Hillery CM (1984) Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia 1984(4):844–852
Ho CCK, Narins PM (2006) Directionality of the pressure-difference receiver ears in the northern leopard frog, Rana pipiens pipiens. J Comp Physiol A 192(4):417–429
Jørgensen MB (1991) Comparative studies of the biophysics of directional hearing in anurans. J Comp Physiol A 169(5):591–598
Jørgensen MB, Christensen-Dalsgaard J (1997a) Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. I. Spike rate responses. J Comp Physiol A 180(5):493–502. doi:10.1007/s003590050066
Jørgensen MB, Christensen-Dalsgaard J (1997b) Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. II. Spike timing. J Comp Physiol A 180(5):503–511. doi:10.1007/s003590050066
Jørgensen MB, Gerhardt HC (1991) Directional hearing in the gray tree frog Hyla versicolor: eardrum vibrations and phonotaxis. J Comp Physiol A 169(2):177–183
Jørgensen MB, Schmitz B, Christensen-Dalsgaard J (1991) Biophysics of directional hearing in the frog Eleutherodactylus coqui. J Comp Physiol A 168(2):223–232
Klump GM, Gerhardt HC (1989) Sound localization in the barking treefrog. Naturwissenschaften 76(1):35–37
Klump GM, Benedix JH, Gerhardt HC, Narins PM (2004) AM representation in green treefrog auditory nerve fibers: neuroethological implications for pattern recognition and sound localization. J Comp Physiol A 190(12):1011–1021
Lindquist ED, Hetherington TE, Volman SF (1998) Biomechanical and neurophysiological studies on audition in eared and earless harlequin frogs (Atelopus). J Comp Physiol A 183(2):265–271. doi:10.1007/s003590050254
Mason MJ (2007) Pathways for sound transmission to the inner ear in amphibians. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer handbook of auditory research, vol 28. Springer, New York, pp 147–183
Melssen WJ, Epping WJM (1992) Selectivity for temporal characteristics of sound and interaural time difference of auditory midbrain neurons in the grassfrog—a system theoretical approach. Hear Res 60(2):178–198. doi:10.1016/0378-5955(92)90020-n
Michelsen A, Jørgensen MB, Christensen-Dalsgaard J, Capranica RR (1986) Directional hearing of awake, unrestrained treefrogs. Naturwissenschaften 73(11):682–683. doi:10.1007/bf00366697
Narins PM, Ehret G, Tautz J (1988) Accessory pathway for sound transfer in a neotropical frog. Proc Natl Acad Sci USA 85(5):1508–1512
Narins PM, Grabul DS, Soma KK, Gaucher P, Hödl W (2005) Cross-modal integration in a dart-poison frog. Proc Natl Acad Sci USA 102(7):2425–2429
Nityananda V, Bee MA (2012) Spatial release from masking in a free-field source identification task by gray treefrogs. Hear Res 285:86–97
Palmer AR, Pinder AC (1984) The directionality of the frog ear described by a mechanical model. J Theor Biol 110(2):205–215. doi:10.1016/s0022-5193(84)80053-3
Penna M, Gormaz JP, Narins PM (2009) When signal meets noise: immunity of the frog ear to interference. Naturwissenschaften 96(7):835–843. doi:10.1007/s00114-009-0542-9
Pinder AC, Palmer AR (1983) Mechanical properties of the frog ear: vibration measurements under free- and closed-field acoustic conditions. Proc R Soc Lond Ser B Biol Sci 219(1217):371–396. doi:10.1098/rspb.1983.0079
Ptacek MB, Gerhardt HC, Sage RD (1994) Speciation by polyploidy in treefrogs: multiple origins of the tetraploid, Hyla versicolor. Evolution 48(3):898–908
Rheinlaender J, Gerhardt HC, Yager DD, Capranica RR (1979) Accuracy of phonotaxis by the green treefrog (Hyla cinerea). J Comp Physiol A 133(4):247–255
Rheinlaender J, Walkowiak W, Gerhardt HC (1981) Directional hearing in the green treefrog: a variable mechanism? Naturwissenschaften 68(8):430–431
Robertson JGM (1986) Male territoriality, fighting and assessment of fighting ability in the Australian frog Uperoleia rugosa. Anim Behav 34:763–772
Schmitz B, White TD, Narins PM (1992) Directionality of phase locking in auditory nerve fibers of the leopard frog Rana pipiens pipiens. J Comp Physiol A 170(5):589–604
Schrode K, Ward JL, Vélez A, Bee MA (2012) Female preferences for spectral call properties in the western genetic lineage of Cope’s gray treefrog (Hyla chrysoscelis). Behav Ecol Sociobiol 66:1595–1606
Schrode KM, Buerkle NP, Brittan-Powell EF, Bee MA (2014) Auditory brainstem responses in Cope’s gray treefrog (Hyla chrysoscelis): Effects of frequency, level, sex and size. J Comp Physiol A. doi:10.1007/s00359-014-0880-8
Schwartz JJ, Gerhardt HC (1989) Spatially mediated release from auditory masking in an anuran amphibian. J Comp Physiol A 166(1):37–41
Schwartz JJ, Gerhardt HC (1995) Directionality of the auditory system and call pattern recognition during acoustic interference in the gray treefrog, Hyla versicolor. Audit Neurosci 1:195–206
Shen JX, Feng AS, Xu ZM, Yu ZL, Arch VS, Yu XJ, Narins PM (2008) Ultrasonic frogs show hyperacute phonotaxis to female courtship calls. Nature 453(7197):914–916
Taylor RC, Klein BA, Stein J, Ryan MJ (2011) Multimodal signal variation in space and time: how important is matching a signal with its signaler? J Exp Biol 214(5):815–820. doi:10.1242/jeb.043638
Ursprung E, Ringler M, Hödl W (2009) Phonotactic approach pattern in the neotropical frog Allobates femoralis: a spatial and temporal analysis. Behaviour 146:153–170. doi:10.1163/156853909x410711
Vlaming MSMG, Aertsen AMHJ, Epping WJM (1984) Directional hearing in the grass frog (Rana temporaria L.): I. Mechanical vibrations of tympanic membrane. Hear Res 14(2):191–201. doi:10.1016/0378-5955(86)90043-2
Wagner WE (1989) Fighting, assessment, and frequency alteration in Blanchard cricket frog. Behav Ecol Sociobiol 25(6):429–436
Wang JX, Narins PM (1996) Directional masking of phase locking in the amphibian auditory nerve. J Acoust Soc Am 99(3):1611–1620
Wang J, Ludwig TA, Narins PM (1996) Spatial and spectral dependence of the auditory periphery in the northern leopard frog. J Comp Physiol A 178(2):159–172
Ward JL, Buerkle NP, Bee MA (2013a) Spatial release from masking improves sound pattern discrimination along a biologically relevant pulse-rate continuum in gray treefrogs. Hear Res 306:63–75
Ward JL, Love EK, Vélez A, Buerkle NP, O’Bryan LR, Bee MA (2013b) Multitasking males and multiplicative females: dynamic signalling and receiver preferences in Cope’s grey treefrog (Hyla chrysoscelis). Anim Behav 86:231–243
Wells KD (1978) Territoriality in the green frog (Rana clamitans): vocalizations and agonistic behavior. Anim Behav 26:1051–1063
White TD, Schmitz B, Narins PM (1992) Directional dependence of auditory sensitivity and frequency selectivity in the leopard frog. J Acoust Soc Am 92(4):1953–1961
Wilczynski W, Brenowitz EA (1988) Acoustic cues mediate inter-male spacing in a neotropical frog. Anim Behav 36:1054–1063
Wilczynski W, Resler C, Capranica RR (1987) Tympanic and extratympanic sound transmission in the leopard frog. J Comp Physiol A 161(5):659–669. doi:10.1007/bf00605007
Acknowledgments
We thank Sandra Tekmen and Jessica Ward for logistical support and for assistance in the lab, Marcos Gridi-Papp for consultation on the development of our experimental methodology, and two anonymous reviewers for their helpful comments on an earlier version of this manuscript. All procedures followed the Guide for the Care and Use of Laboratory Animals and were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (protocol #1103A97192) or the Danish National Animal Experimentation board (protocol 2009/561-1645). This work was supported by a grant from the National Institute on Deafness and Other Communication Disorders (R01 DC009582). Katrina Schrode was supported by a National Institutes of Health Pre-doctoral Training Grant (NIH T32 NS048944).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caldwell, M.S., Lee, N., Schrode, K.M. et al. Spatial hearing in Cope’s gray treefrog: II. Frequency-dependent directionality in the amplitude and phase of tympanum vibrations. J Comp Physiol A 200, 285–304 (2014). https://doi.org/10.1007/s00359-014-0883-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0883-5