Abstract
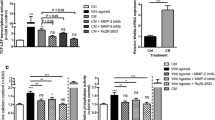
Several cell types contribute to atherosclerotic calcification. Myeloid calcifying cells (MCCs) are monocytes expressing osteocalcin (OC) and bone alkaline phosphatase (BAP). Herein, we tested whether MCCs promote atherosclerotic calcification in vivo. We show that the murine spleen contains OC+BAP+ cells with a phenotype similar to human MCCs, a high expression of adhesion molecules and CD11b, and capacity to calcify in vitro and in vivo. Injection of GFP+ OC+BAP+ cells into 8- or 40-week ApoE−/− mice led to more extensive calcifications in atherosclerotic areas after 24 or 4 weeks, respectively, compared to control OC−BAP− cells. Despite that OC+BAP+ cells had a selective transendothelial migration capacity, tracking of the GFP signal revealed that presence of injected cells within atherosclerotic areas was an extremely rare event and so GFP mRNA was undetectable by qPCR of lesion extracts. By converse, injected OC+BAP+ cells persisted in the bloodstream and bone marrow up to 24 weeks, suggesting a paracrine effect. Indeed, OC+BAP+ cell-conditioned medium (CM) promoted calcification by cultured vascular smooth muscle cells (VSMC) more than CM from OC−BAP− cells. A genomic and proteomic investigation of MCCs identified allograft inflammatory factor (AIF)-1 as a potential candidate of this paracrine activity. AIF-1 stimulated VSMC calcification in vitro and monocyte-specific (CD11b-driven) AIF-1 overexpression in ApoE−/− mice increased calcium content in atherosclerotic areas. In conclusion, we show that murine OC+BAP+ cells correspond to human MCCs and promote atherosclerotic calcification in ApoE−/− mice, through paracrine activity and modulation of resident cells by AIF-1 overexpression.







Similar content being viewed by others
References
Averill MM, Kerkhoff C, Bornfeldt KE (2012) S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 32:223–229. doi:10.1161/ATVBAHA.111.236927
Baars T, Kleinbongard P, Bose D, Konorza T, Mohlenkamp S, Hippler J, Erbel R, Heusch G (2012) Saphenous vein aorto-coronary graft atherosclerosis in patients with chronic kidney disease: more plaque calcification and necrosis, but less vasoconstrictor potential. Basic Res Cardiol 107:303. doi:10.1007/s00395-012-0303-3
Berglund LM, Kotova O, Osmark P, Grufman H, Xing C, Lydrup ML, Goncalves I, Autieri MV, Gomez MF (2012) NFAT regulates the expression of AIF-1 and IRT-1: yin and yang splice variants of neointima formation and atherosclerosis. Cardiovasc Res 93:414–423. doi:10.1093/cvr/cvr309
Cevese A (2012) Totipotent stem cells could do everything or else nothing: the case of vascular reendothelialization. Cardiovasc Res 93:211–212. doi:10.1093/cvr/cvr342
Deb A, Patterson C (2010) Hard luck stories: the reality of endothelial progenitor cells continues to fall short of the promise. Circulation 121:850–852 (pii CIR.0b013e3181d4c360)
Demyanets S, Kaun C, Rychli K, Pfaffenberger S, Kastl SP, Hohensinner PJ, Rega G, Katsaros KM, Afonyushkin T, Bochkov VN, Paireder M, Huk I, Maurer G, Huber K, Wojta J (2011) Oncostatin M-enhanced vascular endothelial growth factor expression in human vascular smooth muscle cells involves PI3K-, p38 MAPK-, Erk1/2- and STAT1/STAT3-dependent pathways and is attenuated by interferon-gamma. Basic Res Cardiol 106:217–231. doi:10.1007/s00395-010-0141-0
Doehring LC, Heeger C, Aherrahrou Z, Kaczmarek PM, Erdmann J, Schunkert H, Ehlers EM (2010) Myeloid CD34+ CD13+ precursor cells transdifferentiate into chondrocyte-like cells in atherosclerotic intimal calcification. Am J Pathol 177:473–480. doi:10.2353/ajpath.2010.090758
Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M (2012) Myocardial infarction accelerates atherosclerosis. Nature 487:325–329. doi:10.1038/nature11260
Egan KP, Kim JH, Mohler ER 3rd, Pignolo RJ (2011) Role for circulating osteogenic precursor cells in aortic valvular disease. Arterioscler Thromb Vasc Biol 31:2965–2971 (pii ATVBAHA.111.234724)
Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, Hakimi M, Bockler D, Katus HA, Gleissner CA (2011) Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol 106:125–134. doi:10.1007/s00395-010-0135-y
Fadini GP, Albiero M, Menegazzo L, Boscaro E, Vigili de Kreutzenberg S, Agostini C, Cabrelle A, Binotto G, Rattazzi M, Bertacco E, Bertorelle R, Biasini L, Mion M, Plebani M, Ceolotto G, Angelini A, Castellani C, Menegolo M, Grego F, Dimmeler S, Seeger F, Zeiher A, Tiengo A, Avogaro A (2011) Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res 108:1112–1121 (pii CIRCRESAHA.110.234088)
Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S (2012) Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation 125:2772–2781 (pii 125/22/2772)
Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145:33–43 (pii S0021-9150(99)00011-8)
Fukui M, Tanaka M, Toda H, Asano M, Yamazaki M, Hasegawa G, Imai S, Fujinami A, Ohta M, Nakamura N (2012) The serum concentration of allograft inflammatory factor-1 is correlated with metabolic parameters in healthy subjects. Metabolism 61:1021–1025. doi:10.1016/j.metabol.2011.12.001
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi:10.1038/nprot.2008.211
Krankel N, Kuschnerus K, Madeddu P, Luscher TF, Landmesser U (2011) A novel flow cytometry-based assay to study leukocyte-endothelial cell interactions in vitro. Cytom A 79:256–262. doi:10.1002/cyto.a.21043
Martinet W, Schrijvers DM, De Meyer GR (2012) Molecular and cellular mechanisms of macrophage survival in atherosclerosis. Basic Res Cardiol 107:297. doi:10.1007/s00395-012-0297-x
Mishima T, Iwabuchi K, Fujii S, Tanaka SY, Ogura H, Watano-Miyata K, Ishimori N, Andoh Y, Nakai Y, Iwabuchi C, Ato M, Kitabatake A, Tsutsui H, Onoe K (2008) Allograft inflammatory factor-1 augments macrophage phagocytotic activity and accelerates the progression of atherosclerosis in ApoE−/− mice. Int J Mol Med 21:181–187
Morohashi T, Iwabuchi K, Watano K, Dashtsoodol N, Mishima T, Nakai Y, Shimada S, Nishida R, Fujii S, Onoe K (2003) Allograft inflammatory factor-1 regulates trinitrobenzene sulphonic acid-induced colitis. Immunology 110:112–119. doi:10.1046/j.1365-2567.2003.01714
Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY (2012) Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res 94:545–554. doi:10.1093/cvr/cvs126
Post S, Abdallah BM, Bentzon JF, Kassem M (2008) Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone 43:32–39 (pii S8756-3282(08)00140-3)
Preusch MR, Rattazzi M, Albrecht C, Merle U, Tuckermann J, Schutz G, Blessing E, Zoppellaro G, Pauletto P, Krempien R, Rosenfeld ME, Katus HA, Bea F (2008) Critical role of macrophages in glucocorticoid driven vascular calcification in a mouse-model of atherosclerosis. Arterioscler Thromb Vasc Biol 28:2158–2164 (pii ATVBAHA.108.174128)
Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME (2005) Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol 25:1420–1425. doi:10.1161/01.ATV.0000166600.58468.1b
Sommerville LJ, Kelemen SE, Ellison SP, England RN, Autieri MV (2012) Increased atherosclerosis and vascular smooth muscle cell activation in AIF-1 transgenic mice fed a high-fat diet. Atherosclerosis 220:45–52. doi:10.1016/j.atherosclerosis.2011.07.095
Sommerville LJ, Xing C, Kelemen SE, Eguchi S, Autieri MV (2009) Inhibition of allograft inflammatory factor-1 expression reduces development of neointimal hyperplasia and p38 kinase activity. Cardiovasc Res 81:206–215. doi:10.1093/cvr/cvn242
Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM (2009) Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res 104:733–741. doi:10.1161/CIRCRESAHA.108.183053
Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM (2001) Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89:1147–1154. doi:10.1161/hh2401.101070
Thorp E, Tabas I (2009) Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol 86:1089–1095 (pii jlb.0209115)
Tian Y, Kelemen SE, Autieri MV (2006) Inhibition of AIF-1 expression by constitutive siRNA expression reduces macrophage migration, proliferation, and signal transduction initiated by atherogenic stimuli. Am J Physiol Cell Physiol 290:C1083–C1091 (pii 00381.2005)
Vasudevan SS, Lopes NH, Seshiah PN, Wang T, Marsh CB, Kereiakes DJ, Dong C, Goldschmidt-Clermont PJ (2003) Mac-1 and Fas activities are concurrently required for execution of smooth muscle cell death by M-CSF-stimulated macrophages. Cardiovasc Res 59:723–733 (pii S0008636303005145)
Yu H, Stoneman V, Clarke M, Figg N, Xin HB, Kotlikoff M, Littlewood T, Bennett M (2011) Bone marrow-derived smooth muscle-like cells are infrequent in advanced primary atherosclerotic plaques but promote atherosclerosis. Arterioscler Thromb Vasc Biol 31:1291–1299 (pii ATVBAHA.110.218578)
Acknowledgments
GPF is supported by a European Foundation for the Study of Diabetes (EFSD)/AstraZeneca young investigator award.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albiero, M., Rattazzi, M., Menegazzo, L. et al. Myeloid calcifying cells promote atherosclerotic calcification via paracrine activity and allograft inflammatory factor-1 overexpression. Basic Res Cardiol 108, 368 (2013). https://doi.org/10.1007/s00395-013-0368-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0368-7