Abstract
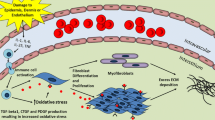
Fibrosis is characterized by the excessive deposition of extracellular matrix components eventually resulting in organ dysfunction and failure. In dermatology, fibrosis is the hallmark component of many skin diseases, including systemic sclerosis, graft-versus-host disease, hypertrophic scars, keloids, nephrogenic systemic fibrosis, porphyria cutanea tarda, restrictive dermopathy and other conditions. Fibrotic skin disorders may be debilitating and impair quality of life. There are few FDA-approved anti-fibrotic drugs; thus, research in this area is crucial in addressing this deficiency. Recent investigations elucidating the pathogenesis of skin fibrosis have implicated endogenous reactive oxygen species produced by the multicomponent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) enzyme complex. In this review, we discuss Nox enzymes and their role in skin fibrosis. An overview of the Nox enzyme family is presented and their role in the pathogenesis of skin fibrosis is discussed. The mechanisms by which Nox enzymes influence specific fibrotic skin disorders are also reviewed. Finally, we describe the therapeutic approaches to ameliorate skin fibrosis by directly targeting Nox enzymes with the use of statins, p47phox subunit modulators, or GKT137831, a competitive inhibitor of Nox enzymes. Nox enzymes can also be targeted indirectly via scavenging ROS with antioxidants. We believe that Nox modulators are worthy of further investigation and have the potential to transform the management of skin fibrosis by dermatologists.






Similar content being viewed by others
Abbreviations
- CCL:
-
Chemokine ligand
- CTGF:
-
Connective tissue growth factor
- ECM:
-
Extracellular matrix
- EGCG:
-
Epigallocatechin-3-gallate
- EMS:
-
Eosinophilia-myalgia syndrome
- ERK:
-
Extracellular signal-regulated kinase
- FAD:
-
Flavin adenine dinucleotide
- GVHD:
-
Graft-versus-host disease
- HSCT:
-
Hematopoietic stem cell transplantation
- IPF:
-
Idiopathic pulmonary fibrosis
- JAK:
-
Janus kinase
- JNK:
-
c-jun amino-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MMP:
-
Matrix metalloproteinase
- mRNA:
-
Messenger RNA
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- Nox:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- NSF:
-
Nephrogenic systemic fibrosis
- PCT:
-
Porphyria cutanea tarda
- PDGF:
-
Platelet-derived growth factor
- PDGFR:
-
Platelet-derived growth factor receptor
- PKC:
-
Protein kinase C
- POLDIP:
-
DNA polymerase delta-interacting protein
- PTP:
-
Protein tyrosine phosphatase
- RAAS:
-
Renin–angiotensin–aldosterone system
- RD:
-
Restrictive dermopathy
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TGF:
-
Transforming growth factor
- TIMP:
-
Tissue inhibitor of metalloproteinase
- TKS:
-
Tyrosine kinase substrate
- TOS:
-
Toxic oil syndrome
- 5-VPTA:
-
N-(5-vinyl-1,3-thiazolidin-2-ylidene)phenylamine
References
Abdullah A, Blakeney P, Hunt R, Broemeling L, Phillips L, Herndon DN, Robson MC (1994) Visible scars and self-esteem in pediatric patients with burns. J Burn Care Rehabil 15(2):164–168
Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, Iida M (2006) Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin–angiotensin system. Hypertens Res Off J Japanese Soc Hypertens 29(10):813–820. doi:10.1291/hypres.29.813
Alexandre J, Hu Y, Lu W, Pelicano H, Huang P (2007) Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res 67(8):3512–3517. doi:10.1158/0008-5472.CAN-06-3914
Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH (2012) The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69(14):2327–2343. doi:10.1007/s00018-012-1010-9
Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA (2012) Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: gKT137831 as a novel potential therapeutic agent. Hepatology 56(6):2316–2327. doi:10.1002/hep.25938
Bailey AJ, Bazin S, Sims TJ, Le Lous M, Nicoletis C, Delaunay A (1975) Characterization of the collagen of human hypertrophic and normal scars. Biochim Biophys Acta 405(2):412–421
Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, Fraticelli P, Sambo P, Funaro A, Kazlauskas A, Avvedimento EV, Gabrielli A (2006) Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 354(25):2667–2676. doi:10.1056/NEJMoa052955
Bataller R, Sancho-Bru P, Gines P, Brenner DA (2005) Liver fibrogenesis: a new role for the renin–angiotensin system. Antioxid Redox Signal 7(9–10):1346–1355. doi:10.1089/ars.2005.7.1346
Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA (2003) NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Investig 112(9):1383–1394. doi:10.1172/JCI18212
Batteux F, Kavian N, Servettaz A (2011) New insights on chemically induced animal models of systemic sclerosis. Curr Opin Rheumatol 23(6):511–518. doi:10.1097/BOR.0b013e32834b1606
Berman B, Duncan MR (1990) Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol 123(3):339–346
Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL (2009) Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Investig 119(8):2359–2365. doi:10.1172/JCI33877
Bleasel NR, Varigos GA (2000) Porphyria cutanea tarda. Australas J Dermatol 41(4):197–206 (quiz 207–208)
Block K, Gorin Y (2012) Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer 12(9):627–637. doi:10.1038/nrc3339
Bloxham DP (1979) The relationship of diphenyleneiodonium-induced hypoglycaemia to the specific covalent modification of NADH-ubiquinone oxidoreductase. Biochem Soc Trans 7(1):103–106
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297(10):433–438. doi:10.1007/s00403-006-0651-7
Bohm F, Edge R, Foley S, Lange L, Truscott TG (2001) Antioxidant inhibition of porphyrin-induced cellular phototoxicity. J Photochem Photobiol B 65(2–3):177–183
Bolognia JL, Jorizzo JL, Schaffer JV (2012) Dermatology, 3rd edn. Mosby, St. Louis
Borthwick LA, Wynn TA, Fisher AJ (2013) Cytokine mediated tissue fibrosis. Biochim Biophys Acta 1832(7):1049–1060. doi:10.1016/j.bbadis.2012.09.014
Brinckmann J, Notbohm H, Tronnier M, Acil Y, Fietzek PP, Schmeller W, Muller PK, Batge B (1999) Overhydroxylation of lysyl residues is the initial step for altered collagen cross-links and fibril architecture in fibrotic skin. J Invest Dermatol 113(4):617–621. doi:10.1046/j.1523-1747.1999.00735.x
Brych SB, Engrav LH, Rivara FP, Ptacek JT, Lezotte DC, Esselman PC, Kowalske KJ, Gibran NS (2001) Time off work and return to work rates after burns: systematic review of the literature and a large two-center series. J Burn Care Rehabil 22(6):401–405
Cao DX, Qiao B, Ge ZQ, Yuan YJ (2004) Comparison of burst of reactive oxygen species and activation of caspase-3 in apoptosis of K562 and HL-60 cells induced by docetaxel. Cancer Lett 214(1):103–113. doi:10.1016/j.canlet.2004.03.047
Chen A, Zhang L, Xu J, Tang J (2002) The antioxidant (−)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem J 368(Pt 3):695–704. doi:10.1042/BJ20020894
Classen JF, Henrohn D, Rorsman F, Lennartsson J, Lauwerys BR, Wikstrom G, Rorsman C, Lenglez S, Franck-Larsson K, Tomasi JP, Kampe O, Vanthuyne M, Houssiau FA, Demoulin JB (2009) Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthr Rheum 60(4):1137–1144. doi:10.1002/art.24381
Cracowski JL, Girolet S, Imbert B, Seinturier C, Stanke-Labesque F, Bessard J, Boignard A, Bessard G, Carpentier PH (2005) Effects of short-term treatment with vitamin E in systemic sclerosis: a double blind, randomized, controlled clinical trial of efficacy based on urinary isoprostane measurement. Free Radic Biol Med 38(1):98–103. doi:10.1016/j.freeradbiomed.2004.09.032
Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D (2005) NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97(9):900–907. doi:10.1161/01.RES.0000187457.24338.3D
De Felice B, Wilson RR, Nacca M (2009) Telomere shortening may be associated with human keloids. BMC Med Genet 10:110. doi:10.1186/1471-2350-10-110
de Souza RB, Macedo AR, Kuruma KA, Macedo PA, Borges CT (2009) Pentoxifylline in association with vitamin E reduces cutaneous fibrosis in systemic sclerosis. Clin Rheumatol 28(10):1207–1212. doi:10.1007/s10067-009-1202-3
Dooley A, Bruckdorfer KR, Abraham DJ (2012) Modulation of fibrosis in systemic sclerosis by nitric oxide and antioxidants. Cardiol Res Pract 2012:521958. doi:10.1155/2012/521958
Dooley A, Shi-Wen X, Aden N, Tranah T, Desai N, Denton CP, Abraham DJ, Bruckdorfer R (2010) Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatology 49(11):2024–2036. doi:10.1093/rheumatology/keq208
Engrav LH, Heimbach DM, Reus JL, Harnar TJ, Marvin JA (1983) Early excision and grafting vs. nonoperative treatment of burns of indeterminant depth: a randomized prospective study. J Trauma 23(11):1001–1004
Fauerbach JA, Heinberg LJ, Lawrence JW, Bryant AG, Richter L, Spence RJ (2002) Coping with body image changes following a disfiguring burn injury. Health Psychol Off J Div Health Psychol Am Psychol Assoc 21(2):115–121
Fauerbach JA, Heinberg LJ, Lawrence JW, Munster AM, Palombo DA, Richter D, Spence RJ, Stevens SS, Ware L, Muehlberger T (2000) Effect of early body image dissatisfaction on subsequent psychological and physical adjustment after disfiguring injury. Psychosom Med 62(4):576–582
Gabrielli A, Svegliati S, Moroncini G, Amico D (2012) New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol J 6:87–95. doi:10.2174/1874312901206010087
Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H (2010) A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5(10):981–993. doi:10.1021/cb100219n
Gill PS, Wilcox CS (2006) NADPH oxidases in the kidney. Antioxid Redox Signal 8(9–10):1597–1607. doi:10.1089/ars.2006.8.1597
Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ (2009) NOX enzymes and pulmonary disease. Antioxid Redox Signal 11(10):2505–2516. doi:10.1089/ARS 2009.2599
Hecker L, Cheng J, Thannickal VJ (2012) Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci 69(14):2365–2371. doi:10.1007/s00018-012-1012-7
Heiskanen KM, Naarala J, Savolainen KM (1995) The effects of N-(5-vinyl-1,3-thiazolidin-2-ylidene)phenylamine (5-VTPA) on the changes in free intracellular calcium and the production of reactive oxygen metabolites in human leukocytes. Toxicology 100(1–3):195–202
Heiskanen KM, Savolainen KM (1997) Effects of 3-phenylamino-1,2-propanediol and its mono- and dioleylesters on the production of reactive oxygen metabolites in human polymorphonuclear leukocytes. Toxicol Lett 91(1):39–45
Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51(2):211–217. doi:10.1161/HYPERTENSIONAHA.107.100214
Hummers LK, Wigley FM (2007) Scleroderma. In: Current rheumatology diagnosis and treatment, 2nd edn. McGraw-Hill, New York
Istok R, Bely M, Stancikova M, Rovensky J (2001) Evidence for increased pyridinoline concentration in fibrotic tissues in diffuse systemic sclerosis. Clin Exp Dermatol 26(6):545–547
Itoh M, Yanaba K, Kobayashi T, Nakagawa H (2007) Taxane-induced scleroderma. Br J Dermatol 156(2):363–367. doi:10.1111/j.1365-2133.2006.07597.x
Jagdeo J, Adams L, Lev-Tov H, Sieminska J, Michl J, Brody N (2010) Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. J Drugs Dermatol 9(12):1523–1526
Jagdeo J, Brody N (2011) Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. J Drugs Dermatol 10(7):753–761
Jinnin M (2010) Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol 37(1):11–25. doi:10.1111/j.1346-8138.2009.00738.x
Jinnin M, Ihn H, Yamane K, Tamaki K (2004) Interleukin-13 stimulates the transcription of the human alpha2(I) collagen gene in human dermal fibroblasts. J Biol Chem 279(40):41783–41791. doi:10.1074/jbc.M406951200
Kashiyama K, Mitsutake N, Matsuse M, Ogi T, Saenko VA, Ujifuku K, Utani A, Hirano A, Yamashita S (2012) miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J Invest Dermatol 132(6):1597–1604. doi:10.1038/jid.2012.22
Kavian N, Marut W, Servettaz A, Laude H, Nicco C, Chereau C, Weill B, Batteux F (2012) Arsenic trioxide prevents murine sclerodermatous graft-versus-host disease. J Immunol 188(10):5142–5149. doi:10.4049/jimmunol.1103538
Keeley FW, Murray RK (2011) The Extracellular matrix. In: Bender DA, Botham KM, Weil PA, Kennelly PJ, Murray RK, Rodwell VW (eds) Harper’s illustrated biochemistry, 29th edn. McGraw-Hill, New York
Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P (2011) NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 21(8):1147–1158. doi:10.1517/13543776.2011.584870
Kim JS, Choi IG, Lee BC, Park JB, Kim JH, Jeong JH, Jeong JH, Seo CH (2012) Neuregulin induces CTGF expression in hypertrophic scarring fibroblasts. Mol Cell Biochem 365(1–2):181–189. doi:10.1007/s11010-012-1258-2
Kisseleva T, Brenner DA (2008) Mechanisms of fibrogenesis. Exp Biol Med 233(2):109–122. doi:10.3181/0707-MR-190
Kitko CL, White ES, Baird K (2012) Fibrotic and sclerotic manifestations of chronic graft-versus-host disease. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 18(Suppl 1):S46–S52. doi:10.1016/j.bbmt.2011.10.021
Klass BR, Branford OA, Grobbelaar AO, Rolfe KJ (2010) The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-beta1-stimulated wound contraction. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc 18(1):80–88. doi:10.1111/j.1524-475X.2009.00552.x
Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhofer S, Radermacher KA, Janssen B, Gorlach A, Schmidt HH (2012) NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J Mol Med 90(12):1391–1406. doi:10.1007/s00109-012-0963-3
Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P (2010) First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53(21):7715–7730. doi:10.1021/jm100773e
Lambeth JD, Krause KH, Clark RA (2008) NOX enzymes as novel targets for drug development. Semin Immunopathol 30(3):339–363. doi:10.1007/s00281-008-0123-6
Last JA, King TE Jr, Nerlich AG, Reiser KM (1990) Collagen cross-linking in adult patients with acute and chronic fibrotic lung disease. Molecular markers for fibrotic collagen. Am Rev Respir Dis 141(2):307–313. doi:10.1164/ajrccm/141.2.307
Lee IT, Yang CM (2012) Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 84(5):581–590. doi:10.1016/j.bcp.2012.05.005
Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, Driscoll B (2009) Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol 296(1):L57–L70. doi:10.1152/ajplung.90411.2008
Leiferman K, Peters M (2012) Eosinophils in cutaneous diseases. In: Fitzpatrick’s dermatology in general medicine, 8th edn. McGraw-Hill, New York
Liu S, Shi-wen X, Abraham DJ, Leask A (2011) CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthr Rheum 63(1):239–246. doi:10.1002/art.30074
Liu X, Zhu S, Wang T, Hummers L, Wigley FM, Goldschmidt-Clermont PJ, Dong C (2005) Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med 2(12):e354. doi:10.1371/journal.pmed.0020354
Lu R, Serrero G (1999) Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol 179(3):297–304. doi:10.1002/(SICI)1097-4652(199906)179:3<297:AID-JCP7>3.0.CO;2-P
Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V (2005) The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 6:11. doi:10.1186/1465-9921-6-11
Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O (2010) MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthr Rheum 62(6):1733–1743. doi:10.1002/art.27443
Mavrikakis ME, Lekakis JP, Papamichael CM, Stamatelopoulos KS, Kostopoulos ChC, Stamatelopoulos SF (2003) Ascorbic acid does not improve endothelium-dependent flow-mediated dilatation of the brachial artery in patients with Raynaud’s phenomenon secondary to systemic sclerosis. Int J Vitam Nutr Res (Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition) 73(1):3–7
Meng M, Li YQ, Yan MX, Kou Y, Ren HB (2007) Effects of epigallocatechin gallate on diethyldithiocarbamate-induced pancreatic fibrosis in rats. Biol Pharm Bull 30(6):1091–1096
Moinzadeh P, Denton C, Krieg T, Black C (2012) Scleroderma. In: Fitzpatrick’s Dermatology in general medicine, 8th edn. McGraw-Hill, New York
Moriguchi T, Fujimoto D (1979) Crosslink of collagen in hypertrophic scar. J Invest Dermatol 72(3):143–145
Mura G, Bhat KM, Pisano A, Licci G, Carta M (2012) Psychiatric symptoms and quality of life in systemic sclerosis. Clini Pract Epidemiol Mental Health 8:30–35. doi:10.2174/1745017901208010030
Murrell DF (1993) A radical proposal for the pathogenesis of scleroderma. J Am Acad Dermatol 28(1):78–85
Nakamura Y, Feng Q, Kumagai T, Torikai K, Ohigashi H, Osawa T, Noguchi N, Niki E, Uchida K (2002) Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J Biol Chem 277(4):2687–2694. doi:10.1074/jbc.M109641200
Park G, Yoon BS, Moon JH, Kim B, Jun EK, Oh S, Kim H, Song HJ, Noh JY, Oh C, You S (2008) Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J Invest Dermatol 128(10):2429–2441. doi:10.1038/jid.2008.103
Pinelli A, Trivulzio S, Tomasoni L, Bertolini B, Pinelli G (2002) High-dose vitamin E lowers urine porphyrin levels in patients affected by porphyria cutanea tarda. Pharmacol Res Off J Italian Pharmacol Soc 45(4):355–359. doi:10.1006/phrs 2002.0956
Pines M, Snyder D, Yarkoni S, Nagler A (2003) Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 9(7):417–425
Proctor CJ, Kirkwood TB (2002) Modelling telomere shortening and the role of oxidative stress. Mech Ageing Dev 123(4):351–363
Rezvani HR, Rossignol R, Ali N, Benard G, Tang X, Yang HS, Jouary T, de Verneuil H, Taieb A, Kim AL, Mazurier F (2011) XPC silencing in normal human keratinocytes triggers metabolic alterations through NOX-1 activation-mediated reactive oxygen species. Biochim Biophys Acta 1807(6):609–619. doi:10.1016/j.bbabio.2010.12.006
Ricard-Blum S, Bresson-Hadni S, Vuitton DA, Ville G, Grimaud JA (1992) Hydroxypyridinium collagen cross-links in human liver fibrosis: study of alveolar echinococcosis. Hepatology 15(4):599–602
Ricard-Blum S, Esterre P, Grimaud JA (1993) Collagen cross-linking by pyridinoline occurs in non-reversible skin fibrosis. Cell Mol Biol 39(7):723–727
Samarakoon R, Overstreet JM, Higgins PJ (2013) TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 25(1):264–268. doi:10.1016/j.cellsig.2012.10.003
Sambo P, Baroni SS, Luchetti M, Paroncini P, Dusi S, Orlandini G, Gabrielli A (2001) Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthr Rheum 44(11):2653–2664
Sampson N, Berger P, Zenzmaier C (2012) Therapeutic targeting of redox signaling in myofibroblast differentiation and age-related fibrotic disease. Oxidative Med Cell Longev 2012:458276. doi:10.1155/2012/458276
Schramm A, Matusik P, Osmenda G, Guzik TJ (2012) Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol 56(5–6):216–231. doi:10.1016/j.vph.2012.02.012
Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, Touyz RM (2012) Oxidative stress, Nox isoforms and complications of diabetes—potential targets for novel therapies. J Cardiovasc Transl Res 5(4):509–518. doi:10.1007/s12265-012-9387-2
Sieprath T, Darwiche R, De Vos WH (2012) Lamins as mediators of oxidative stress. Biochem Biophys Res Commun 421(4):635–639. doi:10.1016/j.bbrc.2012.04.058
Simonini G, Pignone A, Generini S, Falcini F, Cerinic MM (2000) Emerging potentials for an antioxidant therapy as a new approach to the treatment of systemic sclerosis. Toxicology 155(1–3):1–15
Spies-Weisshart B, Schilling K, Bohmer F, Hochhaus A, Sayer HG, Scholl S (2013) Lack of association of platelet-derived growth factor (PDGF) receptor autoantibodies and severity of chronic graft-versus-host disease (GvHD). J Cancer Res Clin Oncol 139(8):1397–1404. doi:10.1007/s00432-013-1451-z
Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR (2007) Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin–angiotensin–aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148(8):3773–3780. doi:10.1210/en.2006-1691
Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, Morawietz H (2006) Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun 344(1):200–205. doi:10.1016/j.bbrc.2006.03.114
Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, Moroncini G, Bacigalupo A, Leoni P, Avvedimento EV, Gabrielli A (2007) Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood 110(1):237–241. doi:10.1182/blood-2007-01-071043
Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, Nakata T (2008) Spironolactone exhibits direct renoprotective effects and inhibits renal renin–angiotensin–aldosterone system in diabetic rats. Eur J Pharmacol 589(1–3):264–271. doi:10.1016/j.ejphar.2008.06.019
Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA (2010) Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 273(1–3):45–52. doi:10.1016/j.tox.2010.04.014
Tsou PS, Talia NN, Pinney AJ, Kendzicky A, Piera-Velazquez S, Jimenez SA, Seibold JR, Phillips K, Koch AE (2012) Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts. Arthr Rheum 64(6):1978–1989. doi:10.1002/art.34336
Uhal BD, Kim JK, Li X, Molina-Molina M (2007) Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des 13(12):1247–1256
Uzawa K, Marshall MK, Katz EP, Tanzawa H, Yeowell HN, Yamauchi M (1998) Altered posttranslational modifications of collagen in keloid. Biochem Biophys Res Commun 249(3):652–655. doi:10.1006/bbrc 1998.8955
van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MM, Middelkoop E, Boers W, Karel Ronday H, DeGroot J, Huizinga TW, Bank RA (2004) Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol J Int Soc Matrix Biol 23(4):251–257. doi:10.1016/j.matbio.2004.06.001
Varga J (2012) Systemic sclerosis (scleroderma) and related disorders. Harrison's Principles of Internal Medicine, 18th edn. McGraw-Hill, New York
Vettori S, Gay S, Distler O (2012) Role of microRNAs in fibrosis. Open Rheumatol J 6:130–139. doi:10.2174/1874312901206010130
Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ (2005) Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J Off Publ Fed Am Soc Exp Biol 19(7):854–856. doi:10.1096/fj.04-2882fje
Wagner B, Tan C, Barnes JL, Ahuja S, Davis TL, Gorin Y, Jimenez F (2012) Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol 181(6):1941–1952. doi:10.1016/j.ajpath.2012.08.026
Wan KC, Evans JH (1999) Free radical involvement in hypertrophic scar formation. Free Radic Biol Med 26(5–6):603–608
Wan KC, Wu HT, Chan HP, Hung LK (2002) Effects of antioxidants on pyridinoline cross-link formation in culture supernatants of fibroblasts from normal skin and hypertrophic scars. Clin Exp Dermatol 27(6):507–512
Wang P, Tang F, Li R, Zhang H, Chen S, Liu P, Huang H (2007) Contribution of different Nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: effect of valsartan. Pharmacol Res Off J Italian Pharmacol Soc 55(5):408–417. doi:10.1016/j.phrs.2007.01.016
Wesche WA, Cutlan RT, Khare V, Chesney T, Shanklin D (2001) Restrictive dermopathy: report of a case and review of the literature. J Cutan Pathol 28(4):211–218
Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR (2008) Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51(2):474–480. doi:10.1161/HYPERTENSIONAHA.107.102467
Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH (2011) NOX1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol 164(3):866–883. doi:10.1111/j.1476-5381.2011.01249.x
Wynn TA (2004) Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4(8):583–594. doi:10.1038/nri1412
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214(2):199–210. doi:10.1002/path.2277
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028–1040. doi:10.1038/nm.2807
Xia L, Wang XX, Hu XS, Guo XG, Shang YP, Chen HJ, Zeng CL, Zhang FR, Chen JZ (2008) Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br J Pharmacol 155(3):387–394. doi:10.1038/bjp.2008.272
Yamamoto A, Ashihara E, Nakagawa Y, Obayashi H, Ohta M, Hara H, Adachi T, Seno T, Kadoya M, Hamaguchi M, Ishino H, Kohno M, Maekawa T, Kawahito Y (2011) Allograft inflammatory factor-1 is overexpressed and induces fibroblast chemotaxis in the skin of sclerodermatous GVHD in a murine model. Immunol Lett 135(1–2):144–150. doi:10.1016/j.imlet.2010.10.015
Yamamoto T (2004) Experimental mouse model of scleroderma:induction by bleomycin. In: Lawrence SC (ed) Animal models of human inflammatory skin diseases. CRC Press, Boca Raton, pp 535–547. doi:10.1684/ejd.2008.0570
Yamamoto T (2006) The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res 297(8):333–344. doi:10.1007/s00403-005-0635-z
Yamamoto T (2009) Scleroderma—pathophysiology. Eur J Dermatol 19(1):14–24. doi:10.1684/ejd 2008.0570
Yamamoto T (2011) Autoimmune mechanisms of scleroderma and a role of oxidative stress. Self/Nonself 2(1):4–10. doi:10.4161/self.2.1.14058
Yamamoto T, Nishioka K (2002) Animal model of sclerotic skin. V: increased expression of alpha-smooth muscle actin in fibroblastic cells in bleomycin-induced scleroderma. Clin Immunol 102(1):77–83. doi:10.1006/clim 2001.5138
Yamamoto T, Takagawa S, Katayama I, Mizushima Y, Nishioka K (1999) Effect of superoxide dismutase on bleomycin-induced dermal sclerosis: implications for the treatment of systemic sclerosis. J Invest Dermatol 113(5):843–847. doi:10.1046/j.1523-1747.1999.00758.x
Yoon HK, Lim JY, Kim TJ, Cho CS, Min CK (2010) Effects of pravastatin on murine chronic graft-versus-host disease. Transplantation 90(8):853–860. doi:10.1097/TP.0b013e3181f2c92b
Yoshizaki A, Yanaba K, Ogawa A, Iwata Y, Ogawa F, Takenaka M, Shimizu K, Asano Y, Kadono T, Sato S (2011) The specific free radical scavenger edaravone suppresses fibrosis in the bleomycin-induced and tight skin mouse models of systemic sclerosis. Arthr Rheum 63(10):3086–3097. doi:10.1002/art.30470
Youn GJ, Uzunyan M, Vachon L, Johnson J, Winder TL, Yano S (2010) Autosomal recessive LMNA mutation causing restrictive dermopathy. Clin Genet 78(2):199–200. doi:10.1111/j.1399-0004.2010.01385.x
Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA (2006) Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55(8):2180–2191. doi:10.2337/db05-1188
Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, Messadi DV, Le AD (2006) Green tea extract and (−)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3 K/AkT signaling pathways. J Invest Dermatol 126(12):2607–2613. doi:10.1038/sj.jid.5700472
Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH (2007) Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen Off Publ Wound Healing Soc Eur Tissue Repair Soc 15(Suppl 1):S32–39. doi:10.1111/j.1524-475X.2007.00223.x
Ziegler TR, Panoskaltsus-Mortari A, Gu LH, Jonas CR, Farrell CL, Lacey DL, Jones DP, Blazar BR (2001) Regulation of glutathione redox status in lung and liver by conditioning regimens and keratinocyte growth factor in murine allogeneic bone marrow transplantation. Transplantation 72(8):1354–1362
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked awards TL1 TR000133 and KL2 TR000134. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R33AI080604.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
O. Babalola and A. Mamalis contributed equally to the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Babalola, O., Mamalis, A., Lev-Tov, H. et al. NADPH oxidase enzymes in skin fibrosis: molecular targets and therapeutic agents. Arch Dermatol Res 306, 313–330 (2014). https://doi.org/10.1007/s00403-013-1416-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-013-1416-8