Abstract
Purpose
To investigate neonatal outcomes and congenital malformations in children born after in vitro fertilization (IVF) and vitrified embryo transfer cycles using human menopausal gonadotrophin and medroxyprogesterone acetate (hMG + MPA) treatment.
Methods
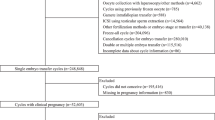
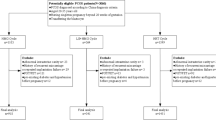
We performed a retrospective cohort study including 4596 live born babies. During January 2014–June 2016, children born after either hMG + MPA treatment, gonadotropin releasing hormone agonist short protocol, or mild ovarian stimulation were included. The main outcome measures were neonatal outcomes and congenital malformations.
Results
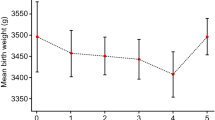
Neonatal outcomes both for singletons and twins such as mean birth weight and length, gestational age, the frequency of preterm birth were comparable between groups. Rate of stillbirth and perinatal death were also similar. No significant differences were found in the overall incidence of congenital malformations between the three groups. Multivariable logistic regression indicated that hMG + MPA regimen did not significantly increase the risk of congenital malformations compared with short protocol and mild ovarian stimulation, with adjusted odds ratio of 1.22 [95% confidence interval (CI) 0.61–2.44] and 1.38 (CI 0.65–2.93), respectively, after adjusting for confounding factors.
Conclusions
Our data suggested that compared with conventional ovarian stimulations, hMG + MPA treatment neither compromised neonatal outcomes of IVF newborns, nor did increase the prevalence of congenital malformations.

Similar content being viewed by others
References
Simpson JL (2014) Birth defects and assisted reproductive technologies. Semin Fetal Neonatal Med 19(3):177–182. doi:10.1016/j.siny.2014.01.001
Yang X, Li Y, Li C, Zhang W (2014) Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril 101(2):385–391. doi:10.1016/j.fertnstert.2013.10.017
Aziz MM, Guirguis G, Maratto S, Benito C, Forman EJ (2016) Is there an association between assisted reproductive technologies and time and complications of the third stage of labor? Arch Gynecol Obstet 293(6):1193–1196. doi:10.1007/s00404-015-3943-3
Santos MA, Kuijk EW, Macklon NS (2010) The impact of ovarian stimulation for IVF on the developing embryo. Reproduction 139(1):23–34. doi:10.1530/rep-09-0187
Ericson A, Kallen B (2001) Congenital malformations in infants born after IVF: a population-based study. Hum Reprod 16(3):504–509. doi:10.1093/humrep/16.3.504
Anthony S, Buitendijk SE, Dorrepaal CA, Lindner K, Braat DD, den Ouden AL (2002) Congenital malformations in 4224 children conceived after IVF. Hum Reprod 17(8):2089–2095. doi:10.1093/humrep/17.8.2089
Boerrigter PJ, de Bie JJ, Mannaerts BM, van Leeuwen BP, Passier-Timmermans DP (2002) Obstetrical and neonatal outcome after controlled ovarian stimulation for IVF using the GnRH antagonist ganirelix. Hum Reprod 17(8):2027–2034. doi:10.1093/humrep/17.8.2027
Bonduelle M, Oberye J, Mannaerts B, Devroey P (2010) Large prospective, pregnancy and infant follow-up trial assures the health of 1000 fetuses conceived after treatment with the GnRH antagonist ganirelix during controlled ovarian stimulation. Hum Reprod 25(6):1433–1440. doi:10.1093/humrep/deq072
Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, Shoham Z (2014) Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril 101(1):105–111. doi:10.1016/j.fertnstert.2013.09.007
Chen H, Wang Y, Lyu Q, Ai A, Fu Y, Tian H, Cai R, Hong Q, Chen Q, Shoham Z, Kuang Y (2015) Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril 103(5):1194–1201. doi:10.1016/j.fertnstert.2015.02.020
Dierschke DJ, Yamaji T, Karsch FJ, Weick RF, Weiss G, Knobil E (1973) Blockade by progesterone of estrogen-induced LH and FSH release in the rhesus monkey. Endocrinology 92(5):1496–1501. doi:10.1210/endo-92-5-1496
Pohl CR, Richardson DW, Marshall G, Knobil E (1982) Mode of action of progesterone in the blockade of gonadotropin surges in the rhesus monkey. Endocrinology 110(4):1454–1455. doi:10.1210/endo-110-4-1454
Richter TA, Robinson JE, Evans NP (2002) Progesterone blocks the estradiol-stimulated luteinizing hormone surge by disrupting activation in response to a stimulatory estradiol signal in the ewe. Biol Reprod 67(1):119–125. doi:10.1095/biolreprod67.1.119
Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, Ai A, Shoham Z (2015) Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril 104(1):62–70. doi:10.1016/j.fertnstert.2015.03.022
Qin N, Chen Q, Hong Q, Cai R, Gao H, Wang Y, Sun L, Zhang S, Guo H, Fu Y, Ai A, Tian H, Lyu Q, Daya S, Kuang Y (2016) Flexibility in starting ovarian stimulation at different phases of the menstrual cycle for treatment of infertile women with the use of in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril 106(2):334–341. doi:10.1016/j.fertnstert.2016.04.006
Massin N (2017) New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update 23(2):211–220. doi:10.1093/humupd/dmw047
Bergh T, Ericson A, Hillensjo T, Nygren KG, Wennerholm UB (1999) Deliveries and children born after in vitro fertilisation in Sweden 1982–95: a retrospective cohort study. Lancet 354(9190):1579–1585. doi:10.1016/s0140-6736(99)04345-7
Westergaard HB, Johansen AM, Erb K, Andersen AN (1999) Danish national in-vitro fertilization registry 1994 and 1995: a controlled study of births, malformations and cytogenetic findings. Hum Reprod 14(7):1896–1902. doi:10.1093/humrep/14.7.1896
Tarlatzis BC, Bili H (2000) Intracytoplasmic sperm injection survey of world results. Ann N Y Acad Sci 900:336–344. doi:10.1111/j.1749-6632.2000.tb06246.x
Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC (2007) Milder ovarian stimulation for in vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 22(4):980–988. doi:10.1093/humrep/del484
Gianaroli L, Magli MC, Cavallini G, Crippa A, Capoti A, Resta S, Robles F, Ferraretti AP (2010) Predicting aneuploidy in human oocytes: key factors which affect the meiotic process. Hum Reprod 25(9):2374–2386. doi:10.1093/humrep/deq123
Fauser BC, Nargund G, Andersen AN, Norman R, Tarlatzis B, Boivin J, Ledger W (2010) Mild ovarian stimulation for IVF: 10 years later. Hum Reprod 25(11):2678–2684. doi:10.1093/humrep/deq247
Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z (2014) Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online 29(6):684–691. doi:10.1016/j.rbmo.2014.08.009
Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF (1986) A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fertil Embryo Transf 3(5):284–295. doi:10.1007/BF01133388
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, van der Poel S (2009) The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod 24(11):2683–2687. doi:10.1093/humrep/dep343
World Health Organization (2016) International statistical classification of diseases and related health problems—10th revision. http://apps.who.int/classifications/icd10/browse/2016/en. Accessed 11 Aug 2016
Belva F, Henriet S, Van den Abbeel E, Camus M, Devroey P, Van der Elst J, Liebaers I, Haentjens P, Bonduelle M (2008) Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod 23(10):2227–2238. doi:10.1093/humrep/den254
Lonergan P (2011) Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76(9):1594–1601. doi:10.1016/j.theriogenology.2011.06.012
Silva CC, Knight PG (2000) Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil 119(2):261–269. doi:10.1530/jrf.0.1190261
Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, Rizos D, Lonergan P (2009) Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 138(3):507–517. doi:10.1530/rep-09-0152
Yamashita Y, Shimada M, Okazaki T, Maeda T, Terada T (2003) Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol Reprod 68(4):1193–1198. doi:10.1095/biolreprod.102.010934
Zhang L, Chen J, Wang Y, Ren F, Yu W, Cheng L (2009) Pregnancy outcome after levonorgestrel-only emergency contraception failure: a prospective cohort study. Hum Reprod 24(7):1605–1611. doi:10.1093/humrep/dep076
Grumbach MM, Ducharme JR, Moloshok RE (1959) On the fetal masculinizing action of certain oral progestins. J Clin Endocrinol Metab 19:1369–1380. doi:10.1210/jcem-19-11-1369
De Santis M, Cavaliere AF, Straface G, Carducci B, Caruso A (2005) Failure of the emergency contraceptive levonorgestrel and the risk of adverse effects in pregnancy and on fetal development: an observational cohort study. Fertil Steril 84(2):296–299. doi:10.1016/j.fertnstert.2005.01.136
Yan J, Huang G, Sun Y, Zhao X, Chen S, Zou S, Hao C, Quan S, Chen ZJ (2011) Birth defects after assisted reproductive technologies in China: analysis of 15,405 offspring in seven centers (2004 to 2008). Fertil Steril 95(1):458–460. doi:10.1016/j.fertnstert.2010.08.024
Belva F, Bonduelle M, Roelants M, Verheyen G, Van Landuyt L (2016) Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum Reprod 31(7):1610–1620. doi:10.1093/humrep/dew103
Wikland M, Hardarson T, Hillensjo T, Westin C, Westlander G, Wood M, Wennerholm UB (2010) Obstetric outcomes after transfer of vitrified blastocysts. Hum Reprod 25(7):1699–1707. doi:10.1093/humrep/deq117
Shi W, Xue X, Zhang S, Zhao W, Liu S, Zhou H, Wang M, Shi J (2012) Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril 97(6):1338–1342. doi:10.1016/j.fertnstert.2012.02.051
Bonduelle M, Mannaerts B, Leader A, Bergh C, Passier D, Devroey P (2012) Prospective follow-up of 838 fetuses conceived after ovarian stimulation with corifollitropin alfa: comparative and overall neonatal outcome. Hum Reprod 27(7):2177–2185. doi:10.1093/humrep/des156
Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C (2013) Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update 19(4):330–353. doi:10.1093/humupd/dmt006
Qin J, Sheng X, Wang H, Liang D, Tan H, Xia J (2015) Assisted reproductive technology and risk of congenital malformations: a meta-analysis based on cohort studies. Arch Gynecol Obstet 292(4):777–798. doi:10.1007/s00404-015-3707-0
Zhu JL, Basso O, Obel C, Bille C, Olsen J (2006) Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ 333(7570):679. doi:10.1136/bmj.38919.495718.AE
Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A (2012) Reproductive technologies and the risk of birth defects. N Engl J Med 366(19):1803–1813. doi:10.1056/NEJMoa1008095
Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, Martikainen H, Tiitinen A, Hartikainen AL (2010) Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Hum Reprod 25(4):914–923. doi:10.1093/humrep/dep477
Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S (2012) Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 98(2):368–377. doi:10.1016/j.fertnstert.2012.05.019
Acknowledgements
We gratefully acknowledge all the staff of the department of assisted reproduction in Shanghai Ninth People’s Hospital for their support and cooperation.
Author information
Authors and Affiliations
Contributions
JZ: Manuscript writing, Data analysis. XM: Manuscript writing, Data analysis. YW: Data collection. QC: Data collection. XL: Data collection. QH: Data collection. YK: Project development.
Corresponding author
Ethics declarations
Funding
This work was supported by grants from National Natural Science Foundation of China (no. 81571397) and Natural Science Foundation of Shanghai (CN) (no. 14411964300).
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Given the retrospective design, written informed consent was not required.
Rights and permissions
About this article
Cite this article
Zhang, J., Mao, X., Wang, Y. et al. Neonatal outcomes and congenital malformations in children born after human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Arch Gynecol Obstet 296, 1207–1217 (2017). https://doi.org/10.1007/s00404-017-4537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4537-z