Abstract
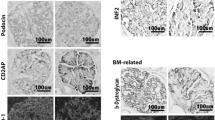
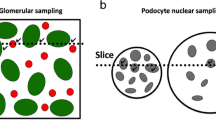
Five different glomerular immunohistochemistry markers were evaluated and compared in four different acute and chronic rat kidney disease models. Progression of glomerular or podocyte damage was shown in the puromycin aminonucleoside nephrosis (PAN) and Zucker fatty/spontaneously hypertensive heart failure F1 hybrid (ZSF1) rat model. Progression and prevention of glomerular damage was demonstrated in the Zucker diabetic fatty (ZDF) and Dahl salt-sensitive (Dahl SS) rat. Immunohistochemistry was performed for desmin, vimentin, podocin, synaptopodin and Wilms tumor protein-1 (WT-1), and evaluation of glomerular immunohistochemistry markers was done by semiautomated quantitative image analysis. We found desmin and WT-1 as the most sensitive markers for podocyte damage in both acute and chronic glomerular damage followed by vimentin, podocin and synaptopodin. We were able to demonstrate that early podocyte damage as shown by increased desmin and vimentin staining together with either a phenotypic podocyte change or podocyte loss (reduced numbers of WT-1-stained podocytes) drives the progression of glomerular damage. This is followed by a reduction in podocyte-specific proteins such as podocin and synaptopodin. Our report describes the different sensitivity of glomerular or podocyte markers and gives future guidance for the selection of the most sensitive markers for efficacy testing of new drugs as well as for the selection of tissue-based toxicity markers for glomerular or podocyte injury. In addition to functional clinical chemistry markers, desmin and WT-1 immunohistochemistry offers reliable and valuable data on the morphologic state of podocytes.






Similar content being viewed by others
References
Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P (2006) Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8:485–491
Barisoni L, Kriz W, Mundel P, D’Agati V (1999) The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10:51–61
Benardeau A, Verry P, Atzpodien EA, Funk JM, Meyer M, Mizrahi J, Winter M, Wright MB, Uhles S, Sebokova E (2013) Effects of the dual PPAR-α/γ agonist aleglitazar on glycaemic control and organ protection in the Zucker diabetic fatty rat. Diabetes Obes Metab 15:164–174
Benigni A, Gagliardini E, Tomasoni S, Abbate M, Ruggenenti P, Kalluri R, Remuzzi G (2004) Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int 65:2000–2193
Bilan VP, Salah EM, Bastacky S, Jones HB, Mayers RM, Zinker B, Poucher SM, Tofovic SP (2011) Diabetic nephropathy and long-term treatment effects of rosiglitazone and enalapril in obese ZSF1 rats. J Endocrinol 210:293–308
Blanco S, Bonet J, López D, Casas I, Romero R (2005) ACE inhibitors improve nephrin expression in Zucker rats with glomerulosclerosis. Kidney Int 67(Suppl 93):S10–S14
Brogan M, Chabanis S, Schwarz SJ, Shankland SJ, Mundel P (2001) Synaptopodin limits podocyte damage in experimental glomerulonephritis (abstract). J Am Soc Nephrol 12:672A–673A
Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J (2000) Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 57:167–182
Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P (2003) Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52:1031–1035
De Boer IH (2012) Chronic kidney disease—a challenge for all ages. JAMA 308:2401–2402
Floege J, Alpers CE, Sage EH, Pritzl P, Gordon K, Johnson RJ, Couser WG (1992) Markers of complement-dependent and complement independent glomerular visceral epithelial cell injury in vivo: expression of antiadhesive proteins and cytoskeletal changes. Lab Invest 67:486–497
Floege J, Hackmann B, Kliem V, Kriz W, Alpers CE, Johnson RJ, Kühn KW, Koch KM, Brunkhorst R (1997) Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int 51:230–243
Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D (2004) Predictors of new-onset kidney disease in a community-based population. JAMA 291:844–850
Fukuda H, Hidaka T, Takagi-Akiba M, Ichimura K, Trejo JA, Sasaki Y, Wang J, Sakai T, Asanuma K, Tomino Y (2015) Podocin is translocated to cytoplasm in puromycin aminonucleoside nephrosis rats and in poor-prognosis patients with IgA nephropathy. Cell Tissue Res 360:391–400
Griffin KA, Abu-Naser M, Abu-Amarah I, Picken M, Williamson GA, Bidani AK (2007) Dynamic blood pressure load and nephropathy in the ZSF1 (fa/fa cp) model of type 2 diabetes. Am J Physiol Renal Physiol 293:F1605–F1613
Gross ML, El-Shakmak A, Szabo A, Koch A, Kuhlmann A, Münter K, Ritz E, Amann K (2003) ACE-inhibitors but not endothelin receptor blockers prevent podocyte loss in early diabetic nephropathy. Diabetologia 46:856–868
Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, for the Alberta Kidney Disease Network (2010) Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303:423–429
Herrmann A, Tozzo E, Funk J (2012) Semi-automated quantitative image analysis of podocyte desmin immunoreactivity as a sensitive marker for acute glomerular damage in the rat puromycin aminonucleoside nephrosis (PAN) model. Exp Toxic Pathol 64:45–49
Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, Nomoto K, Nagata M (2002) Podocyte injury promotes progressive nephropathy in Zucker diabetic fatty rats. Lab Invest 82:25–35
Ishikawa M, Kobayashi N, Sugiyama F, Onoda S, Ishimitsu T (2013) Renoprotective effect of vasopressin v2 receptor antagonist tolvaptan in Dahl rats with end-stage heart failure. Int Heart J 54:98–106
Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM (1991) Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. J Clin Invest 87:847–858
Kakimoto T, Okada K, Hirohashi Y, Relator R, Kawai M, Iguchi T, Fujitaka K, Nishio M, Kato T, Fukunari A, Utsumi H (2014) Automated image analysis of a glomerular injury marker desmin in spontaneously diabetic Torii rats treated with losartan. J Endocrinol 222:43–51
Kakimoto T, Okada K, Fujitaka K, Nishio M, Kato T, Fukunari A, Utsumi H (2015) Quantitative analysis of markers of podocyte injury in the rat puromycin aminonucleoside nephropathy model. Exp Toxicol Pathol 67:171–177
Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F (2003) Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol 13:46–56
Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R (2001) Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60:957–968
Kim J, Shon E, Kim CS, Kim JS (2012) Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res 2012:9 Article ID 210821. http://dx.doi.org/10.1155/2012/210821
Koop K, Eikmans M, Wehland M, Baelde H, Ijpelaar D, Kreutz R, Kawachi H, Kerjaschki D, de Heer E, Bruijn JA (2008) Selective loss of podoplanin protein expression accompanies proteinuria and precedes alterations in podocyte morphology in a spontaneous proteinuric rat model. Am J Pathol 173:315–326
Kriz W, LeHir M (2005) Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int 67:404–419
Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y (2008) Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172:299–308
Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A (2006) Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168:42–54
Menini S, Iacobini C, Oddi G, Ricci C, Simonelli P, Fallucca S, Grattarola M, Pugliese F, Pesce C, Pugliese G (2007) Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia 50:2591–2599
Menke AL, Schedl A (2003) WT1 and glomerular function. Semin Cell Dev Biol 14:233–240
Miyauchi M, Toyoda M, Kobayashi K, Abe M, Kobayashi T, Kato M, Yamamoto N, Kimura M, Umezono T, Suzuki D (2009) Hypertrophy and loss of podocytes in diabetic nephropathy. Intern Med 48:1615–1620
Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W (1997a) Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139:193–204
Mundel P, Reiser J, Kriz W (1997b) Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8:697–705
Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B (1993) Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development 119:1329–1341
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T (2006) Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47:1084–1093
Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, Kashihara N (2010) Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant 25:2889–2898
Omary MB, Coulombe PA, McLean WH (2004) Intermediate filament proteins and their associated diseases. N Engl J Med 351:2087–2100
Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW (1997) Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99:342–348
Pavenstädt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307
Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ (2004) Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286:F606–F616
Schwarz K (2001) Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108:1621–1629
Shankland SJ (2006) The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69:2131–2147
Sofue T, Kiyomoto H, Kobori H, Urushihara M, Nishijima Y, Kaifu K, Hara T, Matsumoto S, Ichimura A, Ohsaki H, Hitomi H, Kawachi H, Hayden MR, Whaley-Connell A, Sowers JR, Ito S, Kohno M, Nishiyama A (2012) Early treatment with olmesartan prevents juxtamedullary glomerular podocyte injury and the onset of microalbuminuria in type 2 diabetic rats. Am J Hypertens 25:604–611
Stamenkovic I, Skalli O, Gabbiani G (1986) Distribution of intermediate filament proteins in normal and diseased human glomeruli. Am J Pathol 125:465–475
Steffes MW, Schmidt D, McCrery R, Basgen JM, for the International Diabetic Nephropathy Study Group (2001) Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59:2104–2113
White KE, Bilous RW (2004) Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant 19:1437–1440
Wiggins RC (2007) The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71:1205–1214
Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC (2005) Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16:2953–2966
Yaoita E, Kawasaki K, Yamamoto T, Kihara I (1990) Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol 136:899–908
Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li Huiping QuZ, Yamamoto T (2006) Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch 448:485–492
Acknowledgments
The authors would like to thank Jacqueline Gillis, Hélène Pierre and Alexander Nuernberg for the careful review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Funk, J., Ott, V., Herrmann, A. et al. Semiautomated quantitative image analysis of glomerular immunohistochemistry markers desmin, vimentin, podocin, synaptopodin and WT-1 in acute and chronic rat kidney disease models. Histochem Cell Biol 145, 315–326 (2016). https://doi.org/10.1007/s00418-015-1391-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-015-1391-6