Abstract
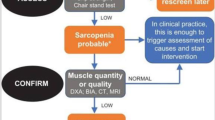
The primary mechanism that contributes to decreasing skeletal muscle strength and size with healthy aging is not presently known. This study examined the contribution of the ubiquitin–proteasome pathway and apoptosis to skeletal muscle wasting in older adults (n = 21; mean age = 72.76 ± 8.31 years) and young controls (n = 21; mean age = 21.48 ± 2.93 years). Subjects underwent a percutaneous muscle biopsy of the vastus lateralis to determine: (1) ubiquitin ligase gene expression (MAFbx and MuRF1); (2) frequency of apoptosis; and (3) individual fiber type and cross-sectional area. In addition, a whole muscle strength test was also performed. A one-way ANOVA revealed significant increases in the number of positive TUNEL cells in older adults (87%; p < 0.05), although no significant increase in caspase-3/7 activity was detected. Additionally, ubiquitin ligase gene expression, individual muscle fiber type and CSA were not different between old and young subjects. Muscle strength was also significantly lower in old compared to young subjects (p < 0.05). In conclusion, this study indicates a preferential role for apoptosis contributing to decreases in muscle function with age.


Similar content being viewed by others
References
Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR (1997) Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273:C579–C587
Argiles JM, Lopez-Soriano FJ (1996) The ubiquitin-dependent proteolytic pathway in skeletal muscle: its role in pathological states. Trends Pharmacol Sci 17:223–226
Bergstrom J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest 14:511–513
Bodine SC, Latres E, Baumhueter S, Lai V, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan Z, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Borisov AB, Carlson BM (2000) Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 258:305–318
Bossola M, Muscaritoli M, Costelli P, Bellantone R, Pacelli F, Busquets S, Argiles J, Lopez-Soriano FJ, Civello IM, Baccino FM, Fanelli FR, Doglietto GB (2001) Increased muscle ubiquitin mRNA levels in gastric cancer patients. Am J Physiol Regul Integr Comp Physiol 280:R1518–R1523
Brooke MH, Kaiser KK (1970) Muscle fiber types: how many and what kind? Arch Neurol 23:369–379
D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R (2003) The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol (Lond) 552:499–511
Deas O, Dumont C, MacFarlane M, Rouleau M, Hebib C, Harper F, Hirsch F, Charpentier B, Cohen CM, Senik A (1998) Caspase-independent cell death induced by anti-cd2 or staurosporine in activated human peripheral t lymphocytes. J Immunol 161:3375–3383
DeMartino G, Ordway GA (1998) Ubiquitin–proteasome pathway of intracellular protein degradation: implications for muscle atrophy during unloading. Exerc Sport Sci Rev 26:219–252
Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER (1993) A survey for assessing physical activity among older adults. Med Sci Sports Exerc 25:628–642
Dirks A, Leeuwenburgh C (2002) Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 282(2):R519–R527
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R (2000) Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88:1321–1326
Hill RJ, Rouillard DB, Chin ER, Ibebunjo C, Wang X, Layfield R, Oleynek JJ (2004) Age-related muscle atrophy is associated with both in increase in poly ubiquitination and expression of the muscle-specific ubiquitin E3 ligase, MAFbx (abstract). FASEB J 18:227–214
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Singh MAF (2001) Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56:B209–B217
Imai Y, Soda M, Takahashi R (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin–protein ligase activity. J Biol Chem 275:35661–35664
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the united states. J Am Geriatr Soc 52:80–85
Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL (2004) Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J 18(9):1025–1027
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li C-Y, Sasaki T, Eila AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong Y, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger J (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549–554
Larsson L, Edstrom L (1986) Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci 76:69–89
Larsson L, Grimby G, Karlsson J (1979) Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol 46:451–456
Larsson L, Li X, Frontera WR (1997) Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272:C638–C649
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? total number, size, and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E, Schoeffler P, Arnal M, Attaix D (1996) Increased mRNA levels for components of the lysosomal, ca2+ -activated, and ATP–ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci USA 93:2714–2718
McCarthy NJ, Whyte MKB, Gilbert CS, Evan GI (1997) Inhibition of ced-3/ice-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the bcl-2 homologue bak. J Cell Biol 136:215–227
Medina R, Wing SS, Goldberg AL (1995) Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J 307:631–637
Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP, Visser M, Health A, and Body Composition Research Group (2003) Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc 51:323–330
Oleynek JJ, Rouillard OB, O’Malley JP, Ibebunjo C, Wang X, Layfield R, Hill RJ (2004) Activation of the ubiquitin–proteasome system in models of muscle wasting (abstract). FASEB J 18:476–478
Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, Franceshi C (1995) Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett 373:291–295
Smith K, Winegard K, Hicks A, McCartney N (2003) Two years of resistance training in older men and women: the effects of three years of detraining on the retention of dynamic strength. Can J Appl Physiol 28:462–474
Tiao G, Hobler S, Wang JJ, Meyer TA, Luchette FA, Fischer JE, Hasselgren P-O (1997) Sepsis is associated with increased mRNAs of the ubiquitin–proteasome proteolytic pathway in human skeletal muscle. J Clin Invest 99:163–168
Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P (2004) Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol (Lond) 557:501–513
Vescovo G, Volteranni M, Zennaro R (2000) Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart 84:431–437
Vescovo G, Zennaro R, Sandri M (1998) Apoptosis of skeletal muscle myofibers and interstitial cells in experimental heart failure. J Mol Cell Cardiol 30:2449–2459
Wagner K-D, Wagner N, Wellmann S, Schley G, Bondke A, Theres H, Scholz H (2003) Oxygen-regulated expression of the Wilms tumor suppressor WT1 involves hypoxia-inducible factor-1 (HIF-1). FASEB J 17(10):1364–1366
Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA (2003) Gene expression profile of aging in human muscle. Physiol Genomics 14:149–159
Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JLW, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH (1999) Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol (Lond) 516:915–930
Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW (2000) Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol 88:627–633
Willoughby DS, Priest JW, Nelson M (2002) Expression of the stress proteins, ubiquitin, heat shock protein 72, and myofibrillar protein content after 12 weeks of leg cycling in person with spinal cord injury. Arch Phys Med Rehabil 83:649–654
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whitman, S.A., Wacker, M.J., Richmond, S.R. et al. Contributions of the ubiquitin–proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch - Eur J Physiol 450, 437–446 (2005). https://doi.org/10.1007/s00424-005-1473-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-005-1473-8