Abstract
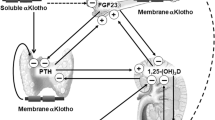
Klotho which was originally identified as an anti-aging protein is emerging as a substance with multiple effects on many systems including mineral homeostasis. In addition to its membrane-bound function as a co-receptor for fibroblast growth factor-23, soluble Klotho exerts effects as a circulating substance in plasma and urine. Novel features of this system include its autocrine–paracrine–endocrine glycan-modifying enzymatic function in the urinary lumen on calcium and phosphate transporters. Klotho induces phosphaturia by inhibiting the proximal tubule Na-coupled phosphate transporter. The action of Klotho is enzymatic in nature which includes alteration of transport activity and the more traditional means of regulation by trafficking. Klotho reduces calciuria by its distal as a sialidase directly on the apical calcium channel. Desialidation of the channel exposes glycan residues that promote binding to galectin-1, resulting in stabilization of residence on the plasma membrane. Through its systematic as well as renal actions, Klotho is emerging as a principal calciophosphoregulatory hormone.





Similar content being viewed by others
References
Agus ZS, Wasserstein A, Goldfarb S (1981) PTH, calcitonin, cyclic nucleotides and the kidney. Annu Rev Physiol 43:583–595. doi:10.1146/annurev.ph.43.030181.003055
Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BC, van Leeuwen JP, Bindels RJ, Hoenderop JG (2009) Klotho prevents renal calcium loss. J Am Soc Nephrol 20(11):2371–2379. doi:10.1681/ASN.2008121273
Barondes SH, Cooper DN, Gitt MA, Leffler H (1994) Galectins. Structure and function of a large family of animal lectins. J Biol Chem 269(33):20807–20810
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117(12):4003–4008. doi:10.1172/JCI32409
Biber J, Hernando N, Forster I, Murer H (2009) Regulation of phosphate transport in proximal tubules. Pflugers Arch 458(1):39–52. doi:10.1007/s00424-008-0580-8
Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C (2009) Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583(19):3221–3224. doi:10.1016/j.febslet.2009.09.009
Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP (2008) A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 105(9):3455–3460. doi:10.1073/pnas.0712361105
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009) Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76(1):38–46. doi:10.1124/mol.109.055780
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105(28):9805–9810
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310(5747):490–493
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104(50):19796–19801. doi:10.1073/pnas.0709805104
Davids MR, Edoute Y, Jungas RL, Cheema-Dhadli S, Halperin ML (2002) Facilitating an understanding of integrative physiology: emphasis on the composition of body fluid compartments. Can J Physiol Pharmacol 80(9):835–850
Demetriou M, Granovsky M, Quaggin S, Dennis JW (2001) Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409(6821):733–739
Ea HK, Liote F (2009) Advances in understanding calcium-containing crystal disease. Curr Opin Rheumatol 21(2):150–157. doi:10.1097/BOR.0b013e3283257ba9
Farrow EG, Davis SI, Summers LJ, White KE (2009) Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol 20(5):955–960. doi:10.1681/ASN.2008070783
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M (2009) FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Ren Physiol 297(2):F282–F291. doi:10.1152/ajprenal.90742.2008
Gattineni J, Baum M (2010) Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol 25(4):591–601. doi:10.1007/s00467-009-1273-z
Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T (2010) Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78(10):975–980. doi:10.1038/ki.2010.313
Hoenderop JG, Muller D, Suzuki M, van Os CH, Bindels RJ (2000) Epithelial calcium channel: gate-keeper of active calcium reabsorption. Curr Opin Nephrol Hypertens 9(4):335–340
Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112(12):1906–1914
Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ (2003) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22(4):776–785
Hofman-Bang J, Martuseviciene G, Santini MA, Olgaard K, Lewin E (2010) Increased parathyroid expression of klotho in uremic rats. Kidney Int 78(11):1119–1127. doi:10.1038/ki.2010.215
Hu M-C, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW (2010) Klotho deficiency is an early biomarker of renal ischemia–reperfusion injury and its replacement is protective. Kidney Int 78(12):1240–1251. doi:http://www.nature.com/ki/journal/v78/n12/suppinfo/ki2010328s1.html
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24(9):3438–3450. doi:10.1096/fj.10-154765
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Shawkat Razzaque M, Rosenblatt KP, Baum MG, Kuro OM, Moe OW (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. doi:10.1096/fj.10-154765
Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro OM, Moe OW (2010) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. doi:10.1681/ASN.2009121311
Huq NL, Cross KJ, Ung M, Reynolds EC (2005) A review of protein structure and gene organisation for proteins associated with mineralised tissue and calcium phosphate stabilisation encoded on human chromosome 4. Arch Oral Biol 50(7):599–609
Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ (2007) A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117(9):2684–2691. doi:10.1172/JCI31330
Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565(1–3):143–147. doi:10.1016/j.febslet.2004.03.090
Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI (2000) Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98(1–2):115–119
Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M (1999) Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest 104(3):229–237. doi:10.1172/JCI5705
Ketteler M, Giachelli C (2006) Novel insights into vascular calcification. Kidney Int Suppl 105:S5–S9. doi:10.1038/sj.ki.5001996
Kuro-o M (2008) Klotho as a regulator of oxidative stress and senescence. Biol Chem 389(3):233–241. doi:10.1515/BC.2008.028
Kuro-o M (2009) Klotho and aging. Biochim Biophys Acta 1790(10):1049–1058. doi:10.1016/j.bbagen.2009.02.005
Kuro-o M (2010) Overview of the FGF23-Klotho axis. Pediatr Nephrol 25(4):583–590. doi:10.1007/s00467-009-1260-4
Kuro-o M (2010) A potential link between phosphate and aging—lessons from Klotho-deficient mice. Mech Ageing Dev 131(4):270–275. doi:10.1016/j.mad.2010.02.008
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51. doi:10.1038/36285
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281(10):6120–6123. doi:10.1074/jbc.C500457200
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M (2005) Suppression of aging in mice by the hormone Klotho. Science 309(5742):1829–1833. doi:10.1126/science.1112766
Lau WL, Festing MH, Giachelli CM (2010) Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost 104(3):464–470. doi:10.1160/TH09-12-0814
Leppanen A, Stowell S, Blixt O, Cummings RD (2005) Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem 280(7):5549–5562. doi:10.1074/jbc.M412019200
Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K (2004) Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29(4):91–99
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317(5839):803–806. doi:10.1126/science.1143578
Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD (2008) FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol 19(12):2342–2350. doi:10.1681/ASN.2007121301
Marks J, Debnam ES, Unwin RJ (2010) Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Ren Physiol 299(2):F285–F296. doi:10.1152/ajprenal.00508.2009
Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M (2005) Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126(12):1274–1283. doi:10.1016/j.mad.2005.07.007
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1998) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242(3):626–630
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367(9507):333–344. doi:10.1016/S0140-6736(06)68071-9
Moe OW (2009) PiT-2 coming out of the pits. Am J Physiol Ren Physiol 296(4):F689–F690. doi:10.1152/ajprenal.00007.2009
Moe OW, Preisig PA (2006) Dual role of citrate in mammalian urine. Curr Opin Nephrol Hypertens 15(4):419–424, 410.1097/1001.mnh.0000232882.0000235469.0000232872
Nancollas GH, LoRe M, Perez L, Richardson C, Zawacki SJ (1989) Mineral phases of calcium phosphate. Anat Rec 224(2):234–241. doi:10.1002/ar.1092240213
Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD (2005) Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123(7):1307–1321. doi:10.1016/j.cell.2005.09.041
Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306(5693):120–124. doi:10.1126/science.1102109
Patel RY, Balaji PV (2006) Identification of linkage-specific sequence motifs in sialyltransferases. Glycobiology 16(2):108–116
Prie D, Friedlander G (2010) Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol 5(9):1717–1722. doi:10.2215/CJN.02680310
Razzaque MS (2009) The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 5(11):611–619. doi:10.1038/nrendo.2009.196
Rennenberg RJ, Schurgers LJ, Kroon AA, Stehouwer CD (2010) Arterial calcifications. J Cell Mol Med 14(9):2203–2210. doi:10.1111/j.1582-4934.2010.01139.x
Robertson WG (1988) Chemistry and biochemistry of calcium. In: Nordin BEC (ed) Calcium in human biology. Human nutrition review edn. Springer, London, pp 1–26
Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC (2009) Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20(11):2348–2358. doi:10.1681/ASN.2009050559
Schauer R (1991) Biosynthesis and function of N- and O-substituted sialic acids. Glycobiology 1(5):449–452
Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K (2007) Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Ren Physiol 292(2):F769–F779. doi:10.1152/ajprenal.00248.2006
Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y (1998) Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424(1–2):6–10
Shiraki-Iida T, Iida A, Nabeshima Y, Anazawa H, Nishikawa S, Noda M, Kuro-o M (2000) Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med 2(4):233–242. doi:10.1002/1521-2254(200007/08)2:4<233::AID-JGM110>3.0.CO;2-5
Sugiura H, Yoshida T, Kohei J (2010) TGF-β was upregulated in renal fibrosis model of Klotho defect mouse and affected renal Klotho expression level (Abstract). J Am Soc Nephrol 21
Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y (2004) Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 109(14):1776–1782. doi:10.1161/01.CIR.0000124224.48962.32
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444(7120):770–774. doi:10.1038/nature05315
Wagner CA, Biber J, Murer H (2007) What goes in must come out—the small intestine modulates renal phosphate excretion. Nephrol Dial Transplant 22(12):3411–3412. doi:10.1093/ndt/gfm554
Williams RJ (2006) The evolution of calcium biochemistry. Biochim Biophys Acta 1763(11):1139–1146. doi:10.1016/j.bbamcr.2006.08.042
Williams RJP (1976) Calcium chemistry and its relation to biological function. Symp Soc Exp Biol 1–17
Yamashita T, Nabeshima Y, Noda M (2000) High-resolution micro-computed tomography analyses of the abnormal trabecular bone structures in klotho gene mutant mice. J Endocrinol 164(2):239–245
Yamashita T, Nifuji A, Furuya K, Nabeshima Y, Noda M (1998) Elongation of the epiphyseal trabecular bone in transgenic mice carrying a klotho gene locus mutation that leads to a syndrome resembling aging. J Endocrinol 159(1):1–8
Yoshida T, Fujimori T, Nabeshima Y (2002) Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 143(2):683–689
Acknowledgments
The authors are supported by the Simmons Family Foundation, the National Institutes of Health (DK081523, AI041612, DK081423, HL096862, DK078708, DK 078596), the O’Brien Center of Kidney Research (DK-079328), and the Charles and Jane Pak Center of Mineral Metabolism.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, CL., Moe, O.W. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch - Eur J Physiol 462, 185–193 (2011). https://doi.org/10.1007/s00424-011-0950-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0950-5