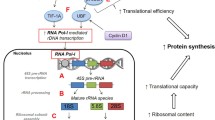
Abstract
How force development and time under tension (TUT) during resistance exercise (RE) influence anabolic signalling of skeletal muscle is incompletely understood. We hypothesized that high force development during RE is more important for post-exercise-induced signalling than submaximal and fatiguing RE with lower force development but similar TUT. Twenty-two male subjects (24 ± 6 years, 181 ± 9 cm, 79 ± 2 kg) performed three distinct RE modes in the fed state with equal TUT but distinct force output: (i) maximal eccentric RE (ECC, n = 7) three sets, eight reps, 100 % eccentric dynamic force; (ii) standard RE (STD, n = 7), three sets, 10 reps, 75 % dynamic force; and (iii) high fatiguing single-set RE (HIT, n = 8), 20 reps, 100 % eccentric-concentric force; vastus lateralis biopsies were collected at baseline, 15, 30, 60, 240 min and 24 h after RE, and the signalling of mechanosensitive and mammalian target of rapamycin (mTOR)-related proteins was determined. The phosphorylation levels of pFAKTyr397, pJNKThr183/Tyr185, pAKTThr308/Ser473, pmTORSer2448, p4E-BP1Thr37/46, p70s6kThr389/Ser421/Thr424 and pS6Ser235/236 were significantly higher in ECC than those in STD and HIT at several time points (P < 0.01). pJNKThr183/Tyr185 and pS6Ser235/236 levels were significantly higher in type II myofibres in ECC compared with STD and HIT. HIT exerted throughout the weakest signalling response. We conclude that high force development during acute RE is superior for anabolic skeletal muscle signalling than fatiguing RE with lower force output but similar TUT. Our results suggest that this response is substantially driven by the higher activation of type II myofibres during RE.






Similar content being viewed by others
Abbreviations
- Nm:
-
Newton meter
- RE:
-
Resistance exercise
- REPS:
-
Repetitions
- TUT:
-
Time under tension
- AKT:
-
v-AKT murine thyoma viral oncogene
- 4E-BP1:
-
Eukaryotic initiation factor 4E-binding protein 1
- MAPK:
-
Mitogen-activated protein kinase
- mTOR:
-
Mammalian target of rapamycin
- JNK:
-
c-jun N-terminal kinase
- p70S6K:
-
70 kDa S6 protein kinase
- p90RSK:
-
90 kDa ribosomal S6 protein kinase
- S6:
-
S6 ribosomal protein
- FAK:
-
Focal adhesion kinase
- AMPK:
-
5-AMP-activated protein kinase
- GSK3-β:
-
Glycogen synthase kinase 3 beta
- TSC-2:
-
Tuberous sclerosis complex 2 (Tuberin)
- CK:
-
Creatine kinase
References
Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ (2005) Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567:1021–1033
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB (2009) Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587:1535–1546
Inoki K, Ouyang H, Zhu TQ, Lindvall C, Wang Y, Zhang XJ, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968
Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS (2005) Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280:7570–7580
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exerciseAm. J Physiol 276:C120–127
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol Lond 576:613–624
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E (2008) Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102:145–152
Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG (2010) Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42:1843–1852
Tannerstedt J, Apro W, Blomstrand E (2009) Maximal lengthening contractions induce different signaling responses in the type I and type II fibers of human skeletal muscle. J Appl Physiol 106:1412–1418
Bird SP, Tarpenning KM, Marino FE (2005) Designing resistance training programmes to enhance muscular fitness: a review of the acute programme variables. Sports Med 35:841–851
Eliasson J, Elfegoun T, Nilsson J, Köhnke R, Ekblom B, Blomstrand E (2006) Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab 291:E1197–1205
Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E (2010) The degree of p70(S6k) and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol 110:835–43
Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM (2010) Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 5:e12033
Martineau LC, Gardiner PF (2002) Skeletal muscle is sensitive to the tension-time integral but not to the rate of change of tension, as assessed by mechanically induced signaling. J Biomech 35:657–663
Klossner S, Durieux AC, Freyssenet D, Flueck M (2009) Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106:389–398
Martineau LC, Gardiner PF (2001) Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol 91:693–702
Nardone A, Schieppati M (1988) Shift of activity from slow to fast muscle during voluntary lengthening contractions of the triceps surae muscles in humans. J Physiol 395:363–381
Gehlert S, Bungartz G, Willkomm L, Korkmaz Y, Pfannkuche K, Schiffer T, Bloch W, Suhr F (2012) Intense resistance exercise induces early and transient increases in ryanodine receptor 1 phosphorylation in human skeletal muscle. PLoS One 7:e49326
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Fridén J, Sjöström M, Ekblom B (1983) Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 4:170–176
Enoka RM (1996) Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol 81:2339–2346
Nardone A, Romanò C, Schieppati M (1989) Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409:451–471
Flück M, Ziemiecki A, Billeter R, Müntener M (2002) Fibre-type specific concentration of focal adhesion kinase at the sarcolemma: influence of fibre innervation and regeneration. J Exp Biol 205:2337–2348
Matsakas A, Patel K (2009) Intracellular signalling pathways regulating the adaptation of skeletal muscle to exercise and nutritional changes. Histol Histopathol 24:209–222
Glass DJ (2003) Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101
Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C (2009) Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. Faseb Journal 23:3896–3905
Koopman R, Zorenc AHG, Gransier RJJ, Cameron-Smith D, van Loon LJC (2006) Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab 290:E1245–E1252
Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ (2008) Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295:E595–604
Liao Y, Hung M-C (2010) Physiological regulation of Akt activity and stability. Am J Transl Res 2:19–42
Blaauw B, Mammucari C, Toniolo L, Agatea L, Abraham R, Sandri M, Reggiani C, Schiaffino S (2008) Akt activation prevents the force drop induced by eccentric contractions in dystrophin-deficient skeletal muscle. Hum Mol Genet 17:3686–3696
Kim CH, Cho YS, Chun YS, Park JW, Kim MS (2002) Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res 90:E25–33
Franchini KG, Torsoni AS, Soares PH, Saad MJ (2000) Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res 87:558–565
Jones JI, Gockerman A, Busby WH, Camacho-Hubner C, Clemmons DR (1993) Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol 121:679–687
Perrone CE, Fenwick-Smith D, Vandenburgh HH (1995) Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J Biol Chem 270:2099–2106
Philp A, Hamilton DL, Baar K (2011) Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol 110:561–568
Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S (2006) The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A 103:4741–4746
You JS, Frey JW, Hornberger TA (2012) Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: implications for a direct activation of mTOR by phosphatidic acid. PLoS One 7:e47258
Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L (1985) Baker SK, and Phillips SM (2012) Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 113:71–77
Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM (2011) Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiologica 201:365–372
Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM (2008) Resistance exercise decreases eIF2Bepsilon phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol 295:R604–610
Shah OJ, Anthony JC, Kimball SR, Jefferson LS (2000) 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279:E715–E729
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273:E99–E107
Pullen N, Thomas G (1997) The modular phosphorylation and activation of p70s6k. FEBS Lett 410:78–82
Ruvinsky I, Meyuhas O (2006) Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31:342–348
Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J (2007) RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem 282:14056–14064
Csukly KJ, Martineau LC, Gardiner PF (2002) Inter- and intra-muscle comparisons of MAPK mechanosensitivity: evidence for the absence of fibre-type dependency. Pflugers Arch 444:732–737
Williamson D, Gallagher P, Harber M, Hollon C, Trappe S (2003) Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. Journal of Physiology-London 547:977–987
Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA (2012) Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS One 7:e37890
Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA (2011) Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25:1028–1039
Bentzinger CF, Lin S, Romanino K, Castets P, Guridi M, Summermatter S, Handschin C, Tintignac LA, Hall MN, Rüegg MA (2013) Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet Muscle 3:6
Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM (2009) Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 107:1655–1662
West DWD, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM (2009) Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. Journal of Physiology-London 587:5239–5247
Acknowledgments
The authors thank Katharina Hermanns and the technicians Anika Voss, Bianca Collins and Mojghan Ghilav (Institute of Cardiovascular Research and Sport Medicine, Department of Molecular and Cellular Sport Medicine) for expert technical assistance.
Conflict of interest
All authors disclose any professional relationship with companies or manufacturers who will benefit from the results of the present study.
Funding
This investigation was funded by the Federal Institute of Sports Science (BISP) IIA1-507 070103/09-10. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 7 Supplementary data
(A) Ratio of phosphorylated pGSK3-β Ser9to α-Tubulin and (B) pTSC-2Thr1462tototal levelsin STD, HIT and ECC. Ratios are from rested fed baseline conditions and 15, 30, 60, 240 min and 24 h after RE. Values are displayed as means ± S.E.M. Data are expressed in arbitrary units (AU). ∗Significantly different from PRE within each exercise group, P < 0.05. § Significantly different from STD within that time point, P < 0.05. # Significantly different from HIT within that time point, P < 0.05. + significantly different between HIT and STD within that time point, P < 0.05. (GIF 63 kb)
Rights and permissions
About this article
Cite this article
Gehlert, S., Suhr, F., Gutsche, K. et al. High force development augments skeletal muscle signalling in resistance exercise modes equalized for time under tension. Pflugers Arch - Eur J Physiol 467, 1343–1356 (2015). https://doi.org/10.1007/s00424-014-1579-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1579-y