Abstract
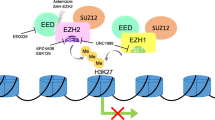
Polycomb group (PcG) proteins are important for the regulation of hematopoiesis by regulating chromatin compaction and silencing genes related to differentiation and cell cycle. Overexpression of enhancer of zeste homologue 2 (Ezh2) and Bmi-1/PCGF4 has been implicated in solid organ cancers, while Mel-18/PCGF2 has been reported as a tumor suppressor. Detailed expression profiles of PcG proteins and their diagnostic significance in malignant lymphomas are still unknown. In this study, we analyzed the expression levels of Ezh2, Bmi-1, Mel-18, and Ki67 in 197 Hodgkin’s and non-Hodgkin’s lymphoma patient samples and in lymphoma cell lines using immunohistochemistry, fluorescent immunocytochemistry, and Western blotting. Immunohistochemical staining showed that Ezh2 expression was significantly increased in aggressive compared to indolent subtypes of B cell neoplasms (P = 0.000–0.030), while no significant differences in Bmi-1 expression were found between these subtypes. Compared to the normal counterpart, T cell lymphomas showed significant overexpression of Bmi-1 (P = 0.011) and Ezh2 (P = 0.000). The Ki67 labeling index showed a positive correlation with Ezh2 expression in B cell lymphomas (correlation coefficient (Co) = 0.983, P = 0.000) and T/NK cell lymphomas (Co = 0.629, P = 0.000). Fluorescent immunohistochemical staining showed coexpression of Ezh2 and Ki67 in the same tumor cells, indicating that Ezh2 expression correlates with cell proliferation. Both B and T/NK cell neoplasms showed low expression of Mel-18 and high expression of both Bmi-1 and Ezh2. In conclusion, in aggressive lymphoma variants, Ezh2 is strongly expressed and polycomb repressive complex PRC1.4 dominates over PRC1.2. Coexpression of Bmi-1 and Ezh2 is a characteristic of aggressive lymphomas. Ezh2 correlates with the proliferation and aggressive nature of non-Hodgkin’s lymphomas.






Similar content being viewed by others
References
Horning SJ, Rosenberg SA (1984) The natural history of initially untreated low-grade non-Hodgkin lymphomas. N Engl J Med 311:1471–1475
Oka T, Ouchida M, Koyama M, Ogama Y, Takada S, Nakatani Y, Tanaka T, Yoshino T, Hayashi K, Ohara N, Kondo E, Takahashi K, Tsuchiyama J, Tanimoto M, Shimizu K, Akagi T (2002) Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res 62:6390–6394
Shaknovich R, Melnick A (2011) Epigenetics and B-cell lymphoma. Curr Opin Hematol 18:293–299
Lessard J, Schumacher A, Sauvageau G (1998) Stage-specific expression of polycomb group genes in human bone marrow cells. Blood 91:1216–1224
Sauvageau M, Sauvageau G (2010) Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Stem Cell 7:299–313
Gao Z, Zhang J, Bonasio R, Strino F, Sawai A, Parisi F, Kluger Y, Reinberg D (2012) PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol Cell 45:344–356
Cao R, Wang L, Wang H, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y (2002) Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 298:1039–1043
Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V (2002) Drosophila enhancer of zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111:185–196
Shao Z, Raible F, Mollaaghababa R, Guyon JR, Wu CT, Bender W, Kingston RE (1999) Stabilization of chromatin structure by PRC1, a polycomb complex. Cell 98:37–46
Wang L, Brown JL, Cao R, Zhang Y, Kassis JA, Jones RS (2004) Hierarchical recruitment of polycomb group silencing complexes. Mol Cell 14:637–646
Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R (2005) BMI-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 19:1432–1437
Cui H, Hu B, Li T, Ma J, Alam G, Gunning WT, Ding HF (2007) BMI-1 is essential for the tumourigenicity of neuroblastoma cells. Am J Pathol 170:1370–1378
Haupt Y, Bath ML, Harris AW, Adams JM (1993) bmi-1 transgene induces lymphomas and collaborates with myc in tumourigenesis. Oncogene 8:3161–3164
Van Galen JC, Muris JJ, Oudejans JJ, Vos W, Giroth CP, Ossenkoppele GJ, Otte AP, Raaphorst FM, Meijer CJ (2007) Expression of the polycomb-group gene BMI-1 is related to an unfavorable prognosis in primary nodal DLBCL. J Clin Pathol 60:167–172
Wang W, Yuasa T, Tsuchiya N, Ma Z, Maita S, Narita S, Kumazawa T, Inoue T, Tsuruta H, Horikawa Y, Saito M, Hu W, Ogawa O, Habuchi T (2009) The novel tumor-suppressor Mel-18 in prostate cancer: its functional polymorphism, expression and clinical significance. Int J Cancer 125:2836–2843
Zhang XW, Sheng YP, Li Q, Qin W, Lu YW, Cheng YF, Liu BY, Zhang FC, Li J, Dimri GP, Guo WJ (2010) BMI1 and Mel-18 oppositely regulate carcinogenesis and progression of gastric cancer. Mol Cancer 9:40
Riis ML, Lüders T, Nesbakken AJ, Vollan HS, Kristensen V, Bukholm IR (2010) Expression of BMI-1 and Mel-18 in breast tissue—a diagnostic marker in patients with breast cancer. BMC Cancer 10:686
Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP (2010) Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis 31:489–495
Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA (2006) EZH2 expression is associated with high proliferation rate and aggressive tumour subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 24:268–273
Wang CG, Ye YJ, Yuan J, Liu FF, Zhang H, Wang S (2010) EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J Gastroenterol 16:2421–2427
Morin RD, Johnson NA, Severson TM et al (2010) Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-centre origin. Nat Genetics 42:181–185
Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, Morin RD, Mungall AJ, Meissner B, Boyle M, Marquez VE, Marra MA, Gascoyne RD, Aparicio SA (2010) Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood 117:2451–2459
Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, Hochhaus A, Drexler HG, Duncombe A, Cervantes F, Oscier D, Boultwood J, Grand FH, Cross NC (2010) Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 42:722–726
Swerdlow SH, Campo E, Harris NL et al (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Hamer KM, Sewalt RG, den Blaauwen JL, Hendrix T, Satijn DP, Otte AP (2002) A panel of monoclonal antibodies against human polycomb group proteins. Hybrid Hybridomics 21:245–252
Sewalt RG, van der Vlag J, Gunster MJ, Hamer KM, den Blaauwen JL, Satijn DP, Hendrix T, van Driel R, Otte AP (1998) Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes. Mol Cell Biol 18:3586–3595
Oka T, Yoshino T, Hayashi K, Ohara N, Nakanishi T, Yamaai Y, Hiraki A, Aoki-Sogawa C, Kondo E, Teramoto N, Takahashi K, Tsuchiyama J, Akagi T (2001) Reduction of hematopoietic cell-specific tyrosine phosphatase SHP-1 gene expression in natural killer cell lymphoma and various types of lymphomas/leukemias: combination analysis with cDNA expression array and tissue microarray. Am J Pathol 159:1495–1505
Raaphorst FM, Otte AP, van Kemenade FJ, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Meijer CJ (2001) Distinct BMI-1 and EZH2 expression patterns in thymocytes and mature T cells suggest a role for polycomb genes in human T cell differentiation. J Immunol 166:5925–5934
Raaphorst FM, van Kemenade FJ, Fieret JH, Satijn DPE, Otto AP, Meijer CJ (2000) Cutting edge: polycomb gene expression patterns reflect distinct B-cell differentiation stages in human germinal centres. J Immunol 164:1–4
Lessard J, Schumacher A, Thorsteinsdottir U, van Lohuizen M, Magnuson T, Sauvageau G (1999) Functional antagonism of the polycomb-group genes eed and Bmi-1 in hemopoietic cell proliferation. Genes Dev 13:2691–2703
Van Lohuizen M (1999) The trithorax groups and Polycomb group chromatin modifiers: implications for disease. Curr Opin Genet Dev 9:355–361
Van Galen JC, Dukers DF, Giroth C, Sewalt RG, Otte AP, Meijer CJ, Raaphorst FM (2004) Distinct expression patterns of polycomb oncoproteins and their binding partners during the germinal center reaction. Eur J Immunol 34:1870–1881
Van Kemenade FJ, Raaphorst FM, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Otte AP, Meijer CJ (2001) Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B-cell non-Hodgkin lymphoma. Blood 97:3896–3901
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns AJM, van Lohuizen M (1999) Bmi-1 collaborates with c-Myc in tumourigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes De 13:2678–2690
Schoeftner S, Sengupta AK, Kubicek S, Mechtler K, Spahn L, Koseki H, Jenuwein T, Wutz A (2006) Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing. EMBO J 25:3110–3122
Sing A, Pannell D, Karaiskakis A, Sturgeon K, Djabali M, Ellis J, Lipshitz HD, Cordes SP (2009) A vertebrate polycomb response element governs segmentation of the posterior hindbrain. Cell 138:885–897
McCabe MT, Ott HM, Ganji G et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492:108–112
Acknowledgments
The authors gratefully acknowledge Drs. M. Maeda (Institute for Virus Research, Kyoto University, Kyoto, Japan), M.J. Robertson (Indiana University School of Medicine, Indianapolis, IN, USA), H. Oono (Kyoto University Medical School, Kyoto, Japan), and Y. Matsuo (Fujisaki Cell Centre, Hayashibara Biochemical Labs, Inc., Okayama, Japan) and RIKEN BioResource Centre (Tsukuba, Japan) for kindly providing the cell lines and also acknowledge Dr. A.P. Otte (University of Amsterdam, Netherlands) for providing anti-Ezh2 antibody. We also thank Mr. H. Okamoto, Mr. K. Isomoto, Ms. H. Nakamura, Ms. M. Bando, Ms. M. Shiotani, and Dr. T. Abdelkader (Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan) for the technical support. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.O.) (#22590312).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd Al Kader, L., Oka, T., Takata, K. et al. In aggressive variants of non-Hodgkin lymphomas, Ezh2 is strongly expressed and polycomb repressive complex PRC1.4 dominates over PRC1.2. Virchows Arch 463, 697–711 (2013). https://doi.org/10.1007/s00428-013-1428-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1428-y