Abstract
Purpose
To retrospectively investigate the optimal regimen of concurrent chemotherapy for nasopharyngeal carcinoma (NPC) by comparing clinical outcomes of patients who received platinum-based and non-platinum-based concurrent chemoradiotherapy (CCRT) regimens.
Methods
Based on a prospectively maintained database from 1998 to 2013 in an endemic area, a total of 4608 newly diagnosed, biopsy-proven, and non-disseminated NPC patients were identified and allocated into three cohorts based on concurrent chemotherapy regimens: cisplatin-based (CP) chemotherapy cohort, other platinum-based (OP) chemotherapy cohort, and non-platinum-based (NP) chemotherapy cohort. Overall survival (OS) and disease-free survival (DFS) were estimated using the Cox proportional hazards model and propensity score analysis of treatment using an inverse probability weighting model (PSA/IPTW). Finally, sensitivity analysis estimated the effects of potential unmeasured confounders.
Results
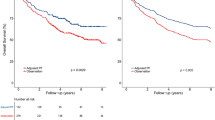
The median follow-up time was 68.5 months (range 2–194 months). The multivariate Cox model showed that NP regimens were significantly related with worse survival compared with CP or OP regimens (OS: HR 1.51, 95% CI 1.16–2.00, P = 0.002; HR 1.68, 95% CI 1.24–2.27, P = 0.001; DFS: HR 1.31, 95% CI 1.03–1.66, P = 0.031; HR 1.50, 95% CI 1.14–1.97, P = 0.004, respectively). Meanwhile, no significant survival difference was found between OP and CP regimens. The PSA/IPTW method, CCRT-specific and III–IVB NPC cohort subgroup analysis showed similar results. Sensitivity analysis confirmed the robustness of our results.
Conclusions
Platinum-based concurrent chemotherapy, including both CP and OP regimens, yields better survival benefits for non-metastatic NPC patients than the NP regimen and remains the optimal regimen for these patients.




Similar content being viewed by others
References
Adelstein D et al (2017) NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Cancer Netw 15:761–770. https://doi.org/10.6004/jnccn.2017.0101
Adham M et al (2012) Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chin J Cancer 31:185–196. https://doi.org/10.5732/cjc.011.10328
Al-Sarraf M et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol 16:1310–1317
Austin PC (2013) The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 32:2837–2849. https://doi.org/10.1002/sim.5705
Blanchard P et al (2015) Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 16:645–655. https://doi.org/10.1016/S1470-2045(15)70126-9
Cao SM, Simons MJ, Qian CN (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30:114–119
Chan AT et al (2005) Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 97:536–539. https://doi.org/10.1093/jnci/dji084
Chen QY et al (2011) Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 103:1761–1770. https://doi.org/10.1093/jnci/djr432
Chen L et al (2012) Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 13:163–171. https://doi.org/10.1016/S1470-2045(11)70320-5
Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Sumitsawan Y, Tharavichitkul E, Sukthomya V, Ford J (2007) Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer 43:1399–1406. https://doi.org/10.1016/j.ejca.2007.03.022
Chua ML, Wee JT, Hui EP, Chan AT (2016) Nasopharyngeal carcinoma. Lancet 387:1012–1024. https://doi.org/10.1016/S0140-6736(15)00055-0
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474. https://doi.org/10.1245/s10434-010-0985-4
Fountzilas G et al (2012) Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol 23:427–435. https://doi.org/10.1093/annonc/mdr116
Gayat E, Resche-Rigon M, Mary JY, Porcher R (2012) Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat 11:222–229. https://doi.org/10.1002/pst.537
Hu W et al (2009) Weekly paclitaxel with concurrent radiotherapy followed by adjuvant chemotherapy in locally advanced nasopharyngeal carcinoma. Radiother Oncol 93:488–491. https://doi.org/10.1016/j.radonc.2009.06.030
Ke LR et al (2017) Safety and efficacy of lobaplatin combined with 5-fluorouracil as first-line induction chemotherapy followed by lobaplatin-radiotherapy in locally advanced nasopharyngeal carcinoma: preliminary results of a prospective phase II trial. BMC Cancer 17:134. https://doi.org/10.1186/s12885-017-3080-4
Kwong DL et al (2004) Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol 22:2643–2653. https://doi.org/10.1200/JCO.2004.05.173
Lee AW et al (2005a) Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 23:6966–6975. https://doi.org/10.1200/JCO.2004.00.7542
Lee AW et al (2005b) Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 61:1107–1116. https://doi.org/10.1016/j.ijrobp.2004.07.702
Lee AW et al (2006) Preliminary results of a randomized study (NPC-9902 trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 66:142–151. https://doi.org/10.1016/j.ijrobp.2006.03.054
Lee AW et al (2011) Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 trials. Eur J Cancer 47:656–666. https://doi.org/10.1016/j.ejca.2010.10.026
Lee AW, Lin JC, Ng WT (2012) Current management of nasopharyngeal cancer. Semin Radiat Oncol 22:233–244. https://doi.org/10.1016/j.semradonc.2012.03.008
Lee AW, Ma BB, Ng WT, Chan AT (2015) Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 33:3356–3364. https://doi.org/10.1200/JCO.2015.60.9347
Lin DY, Psaty BM, Kronmal RA (1998) Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 54:948–963
Ma J et al (2007) Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res 13:1445–1452. https://doi.org/10.1158/1078-0432.CCR-06-2059
Qin J, Ning J, Liu H, Shen Y (2011) Maximum likelihood estimations and EM algorithms with length-biased data. J Am Stat Assoc 106:1434–1449. https://doi.org/10.1198/jasa.2011.tm10156
Sarmiento MP, Mejia MB (2014) Preliminary assessment of nasopharyngeal carcinoma incidence in the Philippines: a second look at published data from four centers. Chin J Cancer 33:159–164. https://doi.org/10.5732/cjc.013.10010
Songthong A, Chakkabat C, Kannarunimit D, Lertbutsayanukul C (2015) Efficacy of intensity-modulated radiotherapy with concurrent carboplatin in nasopharyngeal carcinoma. Radiol Oncol 49:155–162. https://doi.org/10.2478/raon-2014-0044
Sun Y et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17:1509–1520. https://doi.org/10.1016/S1470-2045(16)30410-7
Tang LQ et al (2018) Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II–IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(18)30104-9
Tham IW, Lin S, Pan J, Han L, Lu JJ, Wee J (2010) Intensity-modulated radiation therapy without concurrent chemotherapy for stage IIb nasopharyngeal cancer. Am J Clin Oncol 33:294–299. https://doi.org/10.1097/COC.0b013e3181d2edab
Wee JT, Ha TC, Loong SL, Qian CN (2010) Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer 29:517–526
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ (2014) Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 33:381–387. https://doi.org/10.5732/cjc.014.10086
Wong AS et al (2006) Paclitaxel, 5-fluorouracil and hydroxyurea concurrent with radiation in locally advanced nasopharyngeal carcinoma. Ann Oncol 17:1152–1157. https://doi.org/10.1093/annonc/mdl090
Wu X et al (2013) Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 24:2131–2136. https://doi.org/10.1093/annonc/mdt163
Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, Wu SX (2005) Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol 23:8461–8468. https://doi.org/10.1200/JCO.2004.00.3863
Zhang LN et al (2015) Propensity score matching analysis of cisplatin-based concurrent chemotherapy in low risk nasopharyngeal carcinoma in the intensity-modulated radiotherapy era. Oncotarget. https://doi.org/10.18632/oncotarget.5806
Zhao C et al (2004) Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng 23:1532–1537
Zheng J et al (2010) Induction chemotherapy with nedaplatin with 5-FU followed by intensity-modulated radiotherapy concurrent with chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Jpn J Clin Oncol 40:425–431. https://doi.org/10.1093/jjco/hyp183
Funding
The study was supported by the National Natural Science Foundation of China (Grant nos. 81572665, 81172041, 81472525); the International Cooperation Project of Science and Technology Plan of Guangdong Province (Grant nos. 2014A050503033, 2016A050502011); the Science and Technology Plan Project of Guangdong Province (Grant no. 2013B021800141); and the Foundation of Science and Technology Bureau of Guangzhou City (Grant no. 2014Y2-00179).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. A waiver of informed consent was requested, and approval was obtained from the independent ethics committees at Sun Yat-Sen University Cancer Center.
Availability of data and materials
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the approval number as RDDA2017000419.
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2018_2721_MOESM1_ESM.pdf
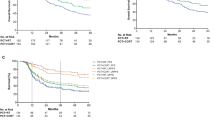
Kaplan-Meier survival curves for the three groups in stage III-IVB NPC cohort. (A) Overall survival, (B) Disease-free survival. NPC, nasopharyngeal carcinoma; CP group, cisplatin-based chemotherapy group; OP group, other platinum-based chemotherapy group; NP group, non-platinum chemotherapy group; CI, confidence interval; HR, hazard ratio (PDF 386 KB)
432_2018_2721_MOESM2_ESM.pdf
Forest plots for overall survival and disease-free survival with hazard ratios and P value by multivariate Cox proportional hazard model and IPTW/PSA in the III-IVB NPC cohort and in the III-IVB NPC, CCRT-specific cohort. NPC, nasopharyngeal carcinoma; CCRT, concurrent chemoradiotherapy; CP group, cisplatin-based chemotherapy group; OP group, other platinum-based chemotherapy group; NP group, non-platinum chemotherapy group; CI, confidence interval; HR, hazard ratio; IPTW/PSA, propensity score analysis of treatment using inverse probability weighting model (PDF 17 KB)
432_2018_2721_MOESM3_ESM.pdf
Kaplan-Meier survival curves for the three groups in stage III-IVB NPC, CCRT-specific cohort. (A) Overall survival, (B) Disease-free survival. NPC, nasopharyngeal carcinoma; CCRT, concurrent chemoradiotherapy; CP group, cisplatin-based chemotherapy group; OP group, other platinum-based chemotherapy group; NP group, non-platinum chemotherapy group; CI, confidence interval; HR, hazard ratio (PDF 376 KB)
Rights and permissions
About this article
Cite this article
Yu, Y., Liang, H., Lv, X. et al. Platinum-based concurrent chemotherapy remains the optimal regimen for nasopharyngeal carcinoma: a large institutional-based cohort study from an endemic area. J Cancer Res Clin Oncol 144, 2231–2243 (2018). https://doi.org/10.1007/s00432-018-2721-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2721-6