Abstract
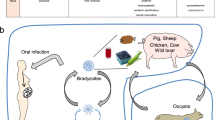
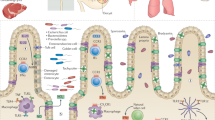
The intracellular apicomplexan parasite Toxoplasma gondii is able to survive and persist in immunocompetent intermediate hosts for the host’s life span. This is despite the induction of a vigorous humoral and—more importantly—cell-mediated immune response during infection. In order to establish and maintain such chronic infections, however, T. gondii has evolved multiple strategies to avoid or to interfere with potentially efficient anti-parasitic immune responses of the host. Such immune evasion includes (1) indirect mechanisms by altering the expression and secretion of immunomodulatory cytokines or by altering the viability of immune cells and (2) direct mechanisms by establishing a lifestyle within a suitable intracellular niche and by interference with intracellular signaling cascades, thereby abolishing a number of antimicrobial effector mechanisms of the host. Despite the parasite’s ability to interfere successfully with the host’s efforts to eradicate the infection, the immune response is, however, not completely abrogated but is rather partially diminished after infection. T. gondii thus keeps a delicate balance between induction and suppression of the host’s immune response in order to guarantee the survival of the host as a safe harbor for parasite development and to allow its transmission to the definitive host.


Similar content being viewed by others
References
Adams LB, Hibbs JB Jr, Taintor RR, Krahenbuhl JL (1990) Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol 144:2725–2729
Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC (2005) Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog 1:e17
Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A (2002a) Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol 3:76–82
Aliberti J, Serhan C, Sher A (2002b) Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med 196:1253–1262
Ambroise-Thomas P, Pelloux H (1993) Toxoplasmosis—congenital and in immunocompromised patients: a parallel. Parasitol Today 9:61–63
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Barragan A, Sibley LD (2002) Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med 195:1625–1633
Barragan A, Sibley LD (2003) Migration of Toxoplasma gondii across biological barriers. Trends Microbiol 11:426–430
Beckers CJM, Dubremetz J-F, Mercereau-Puijalon O, Joiner KA (1994) The Toxoplasma gondii rhoptry protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol 127:947–961
Bermudez LE, Covaro G, Remington J (1993) Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor beta and downregulation of expression of tumor necrosis factor receptors. Infect Immun 61:4126–4130
Bliss SK, Zhang Y, Denkers EY (1999) Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J Immunol 163:2081–2088
Boehm U, Klamp T, Groot M, Howard JC (1997) Cellular responses to interferon-γ. Annu Rev Immunol 15:749–795
Bogdan C, Nathan C (1993) Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci 685:713–739
Bohne W, Heesemann J, Gross U (1994) Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun 62:1761–1767
Bohne W, Holpert M, Gross U (1999) Stage differentiation of the protozoan parasite Toxoplasma gondii. Immunobiology 201:248–254
Butcher BA, Denkers EY (2002) Mechanism of entry determines the ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infect Immun 70:5216–5234
Butcher BA, Kim L, Johnson PF, Denkers EY (2001) Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-κB. J Immunol 167:2193–2201
Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY (2005a) IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-α in host macrophages. J Immunol 174:3148–3152
Butcher BA, Greene RI, Henry SC, Annecharico KL, Weinberg JB, Denkers EY, Sher A, Taylor GA (2005b) p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect Immun 73:3278–3286
Channon JY, Miselis KA, Minns LA, Dutta C, Kasper LH (2002) Toxoplasma gondii induces granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor secretion by human fibroblasts: implications for neutrophil apoptosis. Infect Immun 70:6048–6057
Chao CC, Gekker G, Hu S, Peterson PK (1994) Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol 152:1246–1252
Darnell JE Jr (1997) STATs and gene regulation. Science 277:1630–1635
Däubener W, Remscheid C, Nockemann S, Pilz K, Seghrouchni S, MacKenzie C, Hadding U (1996) Anti-parasitic effector mechanisms in human brain tumor cells: role of interferon-γ and tumor necrosis factor-α. Eur J Immunol 26:487–492
Denkers EY, Gazzinelli RT (1998) Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11:569–588
Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A (1997) Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol 159:1903–1908
Diez B, Galdeano A, Nicolas R, Cisterna R (1989) Relationship between the production of interferon-alpha/beta and interferon-gamma during acute toxoplasmosis. Parasitology 99:11–15
Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 141:2407–2412
Dobbin CA, Smith NC, Johnson AM (2002) Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-κB and nitric oxide. J Immunol 169:958–965
Drapier JC, Wietzerbin J, Hibbs JB Jr (1988) Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol 18:1587–1592
Ellis Neyer L, Grüning G, Fort M, Remington JS, Rennick D, Hunter CA (1997) Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun 65:1675–1682
Ferreira MS, Borges AS (2002) Some aspects of protozoan infections in immunocompromised patients—a review. Mem Inst Oswaldo Cruz 97:443–457
Gavrilescu LC, Denkers EY (2001) IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol 167:902–909
Gavrilescu LC, Denkers EY (2003) Interleukin-12 p40- and Fas ligand-dependent apoptotic pathways involving STAT-1 phosphorylation are triggered during infection with a virulent strain of Toxoplasma gondii. Infect Immun 71:2577–2583
Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A (1991) Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol 146:286–292
Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A (1992) Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149:175–180
Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A (1993) Interleukin 12 is required for the T-lymphocyte-independent induction of interferon-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA 90:6115–6119
Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A (1994) Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153:2533–2543
Gazzinelli RT, Amichay D, Scharton-Kersten T, Grunwald E, Farber JM, Sher A (1996a) Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. In: Gross U (ed) Toxoplasma gondii. Curr Top Microbiol Immunol 219:127–139; Springer, Berlin Heidelberg New York
Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A (1996b) In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol 157:798–805
Ghosh S, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell 109 (Suppl):S81–S96
Goebel S, Lüder CGK, Gross U (1999) Invasion by Toxoplasma gondii protects human-derived HL-60 cells from actinomycin D-induced apoptosis. Med Microbiol Immunol 187:221–226
Goebel S, Gross U, Lüder CGK (2001) Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J Cell Sci 114:3495–3505
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Gubbels M-J, Striepen B, Shastri N, Turkoz M, Robey EA (2005) Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun 73:703–711
Halonen SK, Chiu F, Weiss LM (1998) Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun 66:4989–4993
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Hisaeda H, Sakai T, Ishikawa H, Maekawa Y, Yasutomo K, Good RA, Himeno K (1997) Heat shock protein 65 induced by γδ T cells prevents apoptosis of macrophages and contributes to host defense in mice infected with Toxoplasma gondii. J Immunol 159:2375–2381
Hu MS, Schwartzman JD, Yeaman GR, Collins J, Seguin R, Khan IA, Kasper LH (1999) Fas–FasL interaction involved in pathogenesis of ocular toxoplasmosis in mice. Infect Immun 67:928–935
Hunter CA, Bermudez L, Beernik H, Waegell W, Remington JS (1995) Transforming growth factor-β inhibits interleukin-12-induced production of interferon-γ by natural killer cells: a role for transforming growth factor-β in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur J Immunol 25:994–1000
Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I (1990) Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249:641–646
Jones EY (1997) MHC class I and class II structures. Curr Opin Immunol 9:75–79
Jones TC, Hirsch JG (1972) The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med 136:1173–1194
Kang H, Remington JS, Suzuki Y (2000) Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-γ, TNF-α, and inducible nitric oxide synthase. J Immunol 164:2629–2634
Karin M, Lin A (2002) NF-κB at the crossroads of life and death. Nat Immunol 3:221–227
Keller P, Schaumburg F, Fischer SF, Häcker G, Groß U, Lüder CGK (2006) Direct inhibition of cytochrome c-induced caspase activation in vitro by Toxoplasma gondii reveals novel mechanisms of interference with host cell apoptosis. FEMS Microbiol Lett 258:312–319
Khan I, Matsuura T, Kasper LH (1995) IL-10 mediated immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol 17:185–195
Khan IA, Matsuura T, Kasper LH (1996) Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol 8:887–896
Khan IA, Schwartzman JD, Matsuura T, Kasper LH (1997) A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA 94:13955–13960
Krammer PH (2000) CD95’s deadly mission in the immune system. Nature 407:789–795
Lang C, Algner M, Beinert N, Groß U, Lüder CGK (2006) Diverse mechanisms employed by Toxoplasma gondii to inhibit IFN-γ-induced major histocompatibility complex class II gene expression. Microbes Infect DOI 10.1016/j.micinf.2006.02.031
Langermans JA, Van Der Hulst M E, Nibbering PH, Van Furth R (1992) Endogenous tumor necrosis factor alpha is required for enhanced antimicrobial activity against Toxoplasma gondii and Listeria monocytogenes in recombinant gamma interferon-treated mice. Infect Immun 60:5107–5112
Langermans JAM, Nibbering PH, Van Vuren-Van der Hulst MEB, Van Furth R (2001) Transforming growth factor-β suppresses interferon-γ-induced toxoplasmastatic activity in murine macrophages by inhibition of tumour necrosis factor-α production. Parasite Immunol 23:169–175
Lieberman J (2003) The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 3:361–370
Liesenfeld O, Kosek JC, Suzuki Y (1997) Gamma interferon induces Fas-dependent apoptosis of Peyer’s patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun 65:4682–4689
Lingelbach K, Joiner KA (1998) The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J Cell Sci 111:1467–1475
Lüder CGK, Gross U (2005) Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis. In: Griffin DE (ed) Role of apoptosis in infection. Curr Top Microbiol Immunol 289:219–237; Springer, Berlin Heidelberg New York
Lüder CGK, Seeber F (2001) Toxoplasma gondii and MHC-restricted antigen presentation: on degradation, transport and modulation. Int J Parasitol 31:1355–1369
Lüder CGK, Lang T, Beuerle B, Gross U (1998) Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin Exp Immunol 112:308–316
Lüder CGK, Walter W, Beuerle B, Maeurer MJ, Gross U (2001) Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1α. Eur J Immunol 31:1475–1484
Lüder CGK, Lang C, Giraldo-Velasquez M, Algner M, Gerdes J, Gross U (2003a) Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J Neuroimmunol 134:12–24
Lüder CGK, Algner M, Lang C, Bleicher N, Gross U (2003b) Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int J Parasitol 33:833–844
Luft BJ, Kansas G, Engleman EG, Remington JS (1984) Functional and quantitative alterations in T lymphocyte subpopulations in acute toxoplasmosis. J Infect Dis 150:761–767
Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC (2005) Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLOS Pathog 1:e24
McGavin DDM (1996) Ophthalmology in the topics and subtopics. In: Cook GC (ed) Manson’s tropical diseases. Saunders, London, p 278
McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ (2004) Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J Immunol 173:2632–2640
Medzhitov R, Janeway C Jr (2000) The Toll receptor family and microbial recognition. Trends Microbiol 8:452–456
Mellman I, Turley SJ, Steinman RM (1998) Antigen processing for amateurs and professionals. Trends Cell Biol 8:231–237
Molestina RE, Payne TM, Coppens I, Sinai AP (2003) Activation of NF-κB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IκB to the parasitophorous vacuole membrane. J Cell Sci 116:4359–4371
Mordue DG, Desai N, Dustin M, Sibley LD (1999) Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med 190:1783–1792
Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD (2001) Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167:4574–4584
Murray HW, Juangbhanich CW, Nathan CF, Cohn ZA (1979) Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med 150:950–964
Nash PB, Purner MB, Leon RP, Clarke P, Duke RC, Curiel TJ (1998) Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J Immunol 160:1824–1830
Nelson BH, Willerford DM (1998) Biology of the interleukin-2 receptor. Adv Immunol 70:1–81
Nelson CA, Petzold SJ, Unanue ER (1994) Peptides determine the lifespan of MHC class II molecules in the antigen-presenting cell. Nature 371:250–252
Orlofsky A, Somogyi RD, Weiss LM, Prystowsky MB (1999) The murine antiapoptotic protein A1 is induced in inflammatory macrophages and constitutively expressed in neutrophils. J Immunol 163:412–419
Orlofsky A, Weiss LM, Kawachi N, Prystowsky MB (2002) Deficiency in the anti-apoptotic protein A1-a results in a diminished acute inflammatory response. J Immunol 168:1840–1846
Payne TM, Molestina RE, Sinai AP (2003) Inhibition of caspase activation and a requirement for NF-κB function in the Toxoplasma gondii-mediated blockade of host apoptosis. J Cell Sci 116:4345–4358
Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD, Stark GR (2001) Stat1-independent regulation of gene expression in response to IFN-γ. Proc Natl Acad Sci USA 98:6674–6679
Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A (1997) In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med 186:1819–1829
Richmond A (2002) NF-κB, chemokine gene transcription and tumour growth. Nat Rev Immunol 2:664–674
Rozenfeld C, Martinez R, Figueiredo RT, Bozza MT, Lima FR, Pires AL, Silva PM, Bonomo A, Lannes-Vieira J, De Souza W, Moura-Neto V (2003) Soluble factors released by Toxoplasma gondii-infected astrocytes down-modulate nitric oxide production by gamma interferon-activated microglia and prevent neuronal degeneration. Infect Immun 71:2047–2057
Rozenfeld C, Martinez R, Seabra S, Sant’anna C, Goncalves JG, Bozza M, Moura-Neto V, De Souza W (2005) Toxoplasma gondii prevents neuron degeneration by interferon-γ-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-β1 production by infected microglia. Am J Pathol 167:1021–1031
Savill J, Fadock V (2000) Corpse clearance defines the meaning of cell death. Nature 407:784–788
Sayles PS, Gibson GW, Johnson LL (2000) B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun 68:1026–1033
Scharton-Kersten TM, Yap G, Magram J, Sher A (1997) Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273
Schlüter D, Deckert-Schlüter M, Lorenz E, Meyer T, Röllinghoff M, Bogdan C (1999) Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J Immunol 162:3512–3518
Schwab JC, Beckers CJ, Joiner KA (1994) The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA 91:509–513
Seabra SH, de Souza W, DaMatta RA (2002) Toxoplasma gondii partially inhibits nitric oxide production of activated murine macrophages. Exp Parasitol 100:62–70
Shapira S, Speirs K, Gerstein A, Caamano J, Hunter CA (2002) Suppression of NF-κB activation by infection with Toxoplasma gondii. J Infect Dis 185(Suppl 1):S66–S72
Shapira S, Harb OS, Margarit J, Matrajt M, Han J, Hoffmann A, Freedman B, May MJ, Roos DS, Hunter CA (2005) Initiation and termination of NF-κB signaling by the intracellular protozoan parasite Toxoplasma gondii. J Cell Sci 118:3501–3508
Sher A, Oswald IP, Hieny S, Gazzinelli RT (1993) Toxoplasma gondii induces a T-independent IFN-γ response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol 150:3982–3989
Shrikant P, Benveniste EN (1996) The central nervous system as an immunocompetent organ. Role of glial cells in antigen presentation. J Immunol 157:1819–1822
Shuai K (2000) Modulation of STAT signaling by STAT-interacting proteins. Oncogene 19:2638–2644
Sibley LD, Andrews NW (2000) Cell invasion by un-palatable parasites. Traffic 1:100–106
Sibley LD, Weidner E, Krahenbuhl JL (1985) Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature 315:416–419
Sibley LD, Adams LB, Fukutomi Y, Krahenbuhl JL (1991) Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol 147:2340–2345
Sklenar I, Jones TC, Alkan S, Erb P (1986) Association of symptomatic human infection with Toxoplasma gondii with imbalance of monocytes and antigen-specific T cell subsets. J Infect Dis 153:315–324
Strickland GT, Sayles PC (1977) Depressed antibody responses to a thymus-dependent antigen in toxoplasmosis. Infect Immun 15:184–190
Suzuki Y, Remington JS (1988) Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol 140:3943–3946
Suzuki Y, Orellana MA, Schreiber RD, Remington J S (1988) Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518
Suzuki Y, Sher A, Yap G, Park D, Ellis Neyer L, Liesenfeld O, Fort M, Kang H, Gufwoli E (2000a) IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol 164:5375–5382
Suzuki Y, Kang H, Parmley S, Lim S, Park D (2000b) Induction of tumor necrosis factor-α and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-γ in genetically resistant BALB/c mice. Microbes Infect 2:455–462
Taylor GA, Feng CG, Sher A (2004) p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol 4:100–109
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258
Ting JP-Y, Baldwin AS (1993) Regulation of MHC gene expression. Curr Opin Immunol 5:8–16
Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23:975–1028
Watts C, Amigorena S (2000) Antigen traffic pathways in dendritic cells. Traffic 1:312–317
Williams G (1994) Programmed cell death: a fundamental protective response to pathogens. Trends Microbiol 2:463–464
Yap GS, Sher A (1999) Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med 189:1083–1092
Acknowledgements
We gratefully acknowledge financial support for our investigations by the Deutsche Forschungsgemeinschaft (LU 777/2-1, LU 777/2-2). C. Lang has been supported by a Ph.D. fellowship from the Georg-Lichtenberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, C., Groß, U. & Lüder, C.G.K. Subversion of innate and adaptive immune responses by Toxoplasma Gondii . Parasitol Res 100, 191–203 (2007). https://doi.org/10.1007/s00436-006-0306-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0306-9