Abstract.
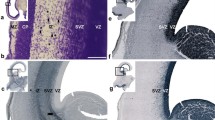
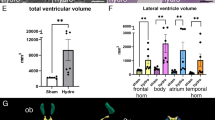
The H-Tx rat has fetal-onset hydrocephalus associated with closure of the cerebral aqueduct and a reduction in the secretory cells of the subcommissural organ (SCO), a circumventricular organ situated in the dorsal wall of the cerebral aqueduct. The objective of this study was to determine the role of the SCO in hydrocephalus pathogenesis. Serial brain sections through aqueduct regions containing the SCO from H-Tx rats, together with non-hydrocephalic Fischer F344 rats, were studied at E16, before hydrocephalus onset, at E17, the beginning of onset, and at P0 when the hydrocephalus was overt. Tissues were immunostained by AFRU, an antibody against the SCO glycoprotein, and for the intermediate filament nestin. The area of SCO cells with AFRU immunostaining and the severity of lateral ventricle dilatation were quantified by image analysis. At E16 all fetuses had distinct SCO ependymal cells, open aqueducts and normal lateral ventricles. The H-Tx fetuses fell into two groups with large areas and small areas of AFRU immunoreactivity, all with a full complement of SCO cells. By E17, fetuses with small areas of immunoreactivity had reduced numbers of tall SCO secretory cells, and most had aqueducts closed posteriorly and dilated ventricles. Three additional fetuses with small areas of immunoreactivity had narrow but patent aqueducts and normal ventricles, and another had an open aqueduct and dilated ventricles. At P0, pups previously identified as hydrocephalic had small areas of AFRU immunoreactivity, an aqueduct that was closed anteriorly but open posteriorly, ventricular dilatation, and an absence of SCO secretory cells. The aqueduct even when closed was lined by typical ependymal cells throughout. Decreased nestin immunostaining accompanied the SCO changes. It is concluded that reduced SCO glycoprotein immunoreactivity precedes both aqueduct closure and expansion of the lateral ventricles in the H-Tx rat.







Similar content being viewed by others
References
Blackshear PJ, Graves JP, Stumpo DJ, Cobos I, Rubenstein JL, Zeldin DC (2003) Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development 130:4539–4552
Boillat CA, Jones HC, Kaiser GL (1999) Aqueduct stenosis in hydrocephalus: ultrastructural investigation in neonatal H-Tx rat brain. Eur J Pediatr Surg 9 Suppl 1:44–46
Boillat CA, Jones HC, Kaiser GL (2001) Inherited hydrocephalus in the H-Tx rat: the ventricular system in late gestation and neonatal aqueduct stenosis. Eur J Pediatr Surg 11 Suppl 1:S43–S44
Bruni JE, Del Bigio MR, Cardoso Er, Persaud TVN (1988) Neuropathology of congenital hydrocephalus in the SUMS/NP mouse. Acta Neurochir 92:118–122
Caprile T, Hein S, Rodríguez S, Montecinos H, Rodríguez E (2003) Reissner fiber binds and transports away monoamines present in the cerebrospinal fluid. Brain Res Mol Brain Res 110:177–192
Castañeyra-Perdomo A, Meyer G, Carmona-Calero E, Bañuelos-Pineda J, Méndez-Medina R, Ormazabal-Ramos C, Ferres-Torres R (1994) Alterations of the subcommissural organ in the hydrocephalic human fetal brain. Brain Res Dev Brain Res 79:316–320
Chouaf-Lakhdar L, Fèvre-Montange M, Brisson C, Strazielle N, Gamrani H, Didier-Bazès M (2003) Proliferative activity and nestin expression in periventricular cells of the adult rat brain. Neuroreport 14:633–636
D’Amato CJ, O’Shea KS, Hicks SP, Glover RA, Annesley TM (1986) Genetic prenatal aqueductal stenosis with hydrocephalus in rat. J Neuropathol Exp Neurol 45:665–682
Danielian PS, McMahon AP (1996) Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature 383:332–334
Estivill-Torrús G, Vitalis T, Fernández-Llebrez P, Price DJ (2001) The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mech Dev 109:215–224
Fukumitsu H, Ohmiya M, Nitta A, Furukawa S, Mima T, Mori K (2000) Aberrant expression of neurotrophic factors in the ventricular progenitor cells of infant congenitally hydrocephalic rats. Childs Nerv Syst 16:516–521
Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, Dastugue B, Meiniel A (1996) SCO-spondin: a new member of the thrombospondin family secreted by the subcommissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci 109:1053–1061
Gobron S, Creveaux I, Meiniel R, Didier R, Herbet A, Bamdad M, El Bitar F, Dastugue B, Meiniel A (2000) Subcommissural organ/Reissner’s fiber complex: characterization of SCO-spondin, a glycoprotein with potent activity on neurite outgrowth. Glia 32:177–191
Grondona JM, Pérez-Martín M, Cifuentes M, Pérez J, Jiménez AJ, Pérez-Fígares JM, Fernández-Llebrez P (1996) Ependymal denudation, aqueductal obliteration and hydrocephalus after a single injection of neuraminidase into the lateral ventricle of adult rats. J Neuropathol Exp Neurol 55:999–1008
Hansske B, Thiel C, Lübke T, Hasilik M, Höning S, Peters V, Heidemann PH, Hoffmann GF, Berger EG, von Figura K, Korner C (2002) Deficiency of UDP-galactose:N-acetylglucosamine beta-1, 4-galactosyltransferase I causes the congenital disorder of glycosylation type IId. J Clin Invest 109:725–733
Jaeken J (2003) Komrower lecture. Congenital disorders of glycosylation (CDG): it’s all in it! J Inherit Metab Dis 26:99–118
Jiménez AJ, Tomé M, Paéz P, Wagner C, Rodríguez S, Fernández-Llebrez P, Rodríguez EM, Pérez-Fígares JM (2001) A programmed ependymal denudation precedes congenital hydrocephalus in the hyh mutant mouse. J Neuropathol Exp Neurol 60:1105–1119
Jones HC (1993) Physiology of cerebrospinal fluid circulation: amphibians, mammals, and hydrocephalus. In: Oksche A, Rodríguez EM, Fernández-Llebrez P (eds) The subcommissural organ. An ependymal brain gland. Springer-Verlag, Berlin, pp 243–253
Jones HC, Bucknall RM (1987) Changes in cerebrospinal fluid pressure and outflow from the lateral ventricles during development of congenital hydrocephalus in the H-Tx rat. Exp Neurol 98:573–583
Jones HC, Bucknall RM (1988) Inherited prenatal hydrocephalus in the H-Tx rat: a morphological study. Neuropathol Appl Neurobiol 14:263–274
Jones HC, Sellars RA (1982) The movement of fluid out of the cerebral ventricles in fetal and neonatal rats. Z Kinderchir 37:130–133
Jones HC, Dack S, Ellis C (1987) Morphological aspects of the development of hydrocephalus in a mouse mutant (SUMS/NP). Acta Neuropathol (Berl) 72:268–276
Jones HC, Bucknall RM, Harris NG (1991) The cerebral cortex in congenital hydrocephalus in the H-Tx rat: a quantitative light microscopy study. Acta Neuropathol (Berl) 82:217–224
Jones HC, Carter BJ, Depelteau JS, Roman M, Morel L (2001a) Chromosomal linkage associated with disease severity in the hydrocephalic H-Tx rat. Behav Genet 31:101–111
Jones HC, Depelteau JS, Carter BJ, Lopman BA, Morel L (2001b) Genome-wide linkage analysis of inherited hydrocephalus in the H-Tx rat. Mamm Genome 12:22–26
Jones HC, Depelteau JS, Carter BJ, Somera KC (2002) The frequency of inherited hydrocephalus is influenced by intrauterine factors in H-Tx rats. Exp Neurol 176:213–220
Kohn DF, Chinookoswong N, Chou SM (1981) A new model of congenital hydrocephalus in the rat. Acta Neuropathol 54:211–218
Louvi A, Wassef M (2000) Ectopic engrailed 1 expression in the dorsal midline causes cell death, abnormal differentiation of circumventricular organs and errors in axonal pathfinding. Development 127:4061–4071
Mashayekhi F, Draper CE, Bannister CM, Pourghasem M, Owen-Lynch PJ, Miyan JA (2002) Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain 125:1859–1874
Meiniel A (2001) SCO-spondin, a glycoprotein of the subcommissural organ/Reissner’s fiber complex: evidence of a potent activity on neuronal development in primary cell cultures. Microsc Res Tech 52:484–495
Miranda E, Almonacid JA, Rodríguez S, Pérez J, Hein S, Cifuentes M, Fernández-Llebrez P, Rodríguez EM (2001) Searching for specific binding sites of the secretory glycoproteins of the subcommissural organ. Microsc Res Tech 52:541–551
Nojima Y, Enzan H, Hayashi Y, Nakayama H, Kiyoku M, Mori K (1998) Neuroepithelial and ependymal changes in HTX rats with congenital hydrocephalus: an ultrastructural and immunohistochemical study. Pathol Int 48:115–125
Overholzer MD, Whitley JR, O’Dell BL, Hogan AG (1954) The ventricular system in hydrocephalic rat brains produced by a deficiency of vitamin B12 or of folic acid in the maternal diet. Anat Rec 120:917–933
Owen-Lynch PJ, Draper CE, Mashayekhi F, Bannister CM, Miyan JA (2003) Defective cell cycle control underlies abnormal cortical development in the hydrocephalic Texas rat. Brain 126:623–631
Pérez-Fígares JM, Jiménez DF, Pérez-Martín M, Fernández-Llebrez P, Cifuentes M, Riera P, Rodríguez S, Rodríguez EM (1998) Spontaneous congenital hydrocephalus in the mutant mouse hyh. Changes in the ventricular system and the subcommissural organ. J Neuropathol Exp Neurol 57:188–202
Pérez-Fígares JM, Jiménez AJ, Rodríguez EM (2001) Subcommissural organ, cerebrospinal fluid circulation, and hydrocephalus. Microsc Res Tech 52:591–607
Pourghasem M, Mashayekhi F, Bannister CM, Miyan J (2001) Changes in the CSF fluid pathways in the developing rat fetus with early onset hydrocephalus. Eur J Pediatr Surg 11 Suppl 1:S10–S13
Rakic P, Sidman RL (1968) Subcommissural organ and adjacent ependyma: autoradiographic study of their origin in the mouse brain. Am J Anat 122:317–335
Renier D, Sainte-Rose C, Pierre-Kahn A, Hirsch JF (1988) Prenatal hydrocephalus: outcome and prognosis. Childs Nerv Syst 4:213–222
Rodríguez EM, Oksche A, Hein S, Rodríguez S, Yulis R (1984) Comparative immunocytochemical study of the subcommissural organ. Cell Tissue Res 237:427–441
Rodríguez EM, Jara P, Richter H, Montecinos H, Flández B, Wiegand R, Oksche A (1993) Evidence for the release of CSF-soluble secretory material from the subcommissural organ, with particular reference to the situation in the human. In: Oksche A, Rodríguez EM, Fernández-Llebrez P (eds) The subcommissural organ. An ependymal brain gland. Springer-Verlag, Berlin, pp 121–131
Rodríguez EM, Rodríguez S, Hein S (1998) The subcommissural organ. Microsc Res Tech 41:98–123
Rodríguez EM, Oksche A, Montecinos H (2001) Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Tech 52:573–590
Sakakibara S, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, Ueda S, Uchiyama Y, Noda T, Okano H (2002) RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci U S A 99:15194–15199
Schöbitz K, Rodríguez EM, Garrido O, del Brio-Leon MA (1993) Ontogenetic development of the subcommissural organ with reference to the flexural organ. In: Oksche A, Rodríguez EM, Fernández-Llebrez P (eds) The subcommissural organ. An ependymal brain gland. Springer-Verlag, Berlin, pp 41–49
Schöniger S, Maronde E, Kopp MD, Korf HW, Nürnberger F (2002) Transcription factor CREB and its stimulus-dependent phosphorylation in cell and explant cultures of the bovine subcommissural organ. Cell Tissue Res 308:131–142
Shi M, Wei LC, Cao R, Chen LW (2002) Enhancement of nestin protein-immunoreactivity induced by ionizing radiation in the forebrain ependymal regions of rats. Neurosci Res 44:475–481
Takano T, Rutka JT, Becker LE (1996) Overexpression of nestin and vimentin in ependymal cells in hydrocephalus. Acta Neuropathol 92:90–97
Takeuchi IK, Kimura R, Matsuda M, Shoji R (1987) Absence of subcommissural organ in the cerebral aqueduct of congenital hydrocephalus spontaneously occurring in MT/HOK1 dr mice. Acta Neuropathol (Berl) 73:320–322
Takeuchi IK, Kimura R, Shoji R (1988) Dysplasia of subcommissural organ in congenital hydrocephalus spontaneously occurring in CWS/Idr rats. Experientia 44:338–340
Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y (2001) Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol 433:62–74
Vio K, Rodríguez S, Navarrete EH, Pérez-Fígares JM, Jiménez AJ, Rodríguez EM (2000) Hydrocephalus induced by immunological blockage of the subcommissural organ-Reissner’s fiber (RF) complex by maternal transfer of anti-RF antibodies. Exp Brain Res 135:41–52
Wada M (1988) Congenital hydrocephalus in HTX-rats: incidence, pathogenesis and developmental impairment. Neurol Med Chir 28:955–964
Wagner C, Batiz LF, Rodríguez S, Jiménez AJ, Paéz P, Tomé M, Pérez-Fígares JM, Rodríguez EM (2003) Cellular mechanisms involved in the stenosis and obliteration of the cerebral aqueduct of hyh mutant mice developing congenital hydrocephalus. J Neuropath Exp Neurol (in press)
Wei LC, Shi M, Chen LW, Cao R, Zhang P, Chan YS (2002) Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res Dev Brain Res 139:9–17
Woodard JC, Newberne PM (1967) The pathogenesis of hydrocephalus in newborn rats deficient in vitamin B12. J Embryol Exp Morphol 17:177–187
Yamada H, Oi S, Tamaki N, Matsumoto S, Sudo K (1992) Histological changes in the midbrain around the aqueduct in congenital hydrocephalic rat LEW/Jms. Childs Nerv Syst 8:394–398
Acknowledgements.
We thank E.M. Rodríguez for providing the AFRU antibody and Logan Wolpin for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding was provided by the National Institutes of Health (NS40359). K.C.S. was supported by the University of Florida Scholars Program and Sigma Xi Grants-in-Aid
Rights and permissions
About this article
Cite this article
Somera, K.C., Jones, H.C. Reduced subcommissural organ glycoprotein immunoreactivity precedes aqueduct closure and ventricular dilatation in H-Tx rat hydrocephalus. Cell Tissue Res 315, 361–373 (2004). https://doi.org/10.1007/s00441-003-0843-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0843-9