Abstract
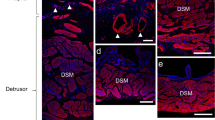
The urothelium plays a sensory role responding to deformation of the bladder wall; this involves the release of adenosine trisphosphate (ATP) and nitric oxide (NO), which affect afferent nerve discharge and bladder sensation. The urothelial cells responsible for producing ATP and NO and the cellular targets, other than afferent nerves, for ATP and NO remain largely unexplored. Sub-urothelial interstitial cells (SU-ICs) lie immediately below the urothelium and respond to NO with a rise in cGMP. To determine which cells might target SU-ICs by producing NO, areas of dome, lateral wall and base wall were treated with isobutyl-methyl-xanthine, exposed to the NO donor diethylamino NONOate and then fixed for immunohistochemistry. Surface urothelial cells (SUCs) in the base and dome expressed neuronal nitric oxide synthase (nNOS), whereas those in the lateral wall did not. Distinct populations of SUCs were present in the bladder base. SUCs with significant amounts of nNOS lay adjacent to cells with low levels of nNOS. In specific base regions, the few SUCs present contained nNOS within discrete intracellular particles. In the basal urothelial cell (BUC) layer of the lateral wall, nNOS-positive (NOS+) BUCs neither showed an elevation in cGMP in response to NO, nor expressed the β1 sub-unit of soluble guanylate cyclase, protein kinase I or protein kinase II. Thus, they produced but did not respond to NO. The BUC layer also stained for the stem cell factor c-Kit suggesting its involvement in urothelial cell development. No NOS+ BUCs were present in the SUC-sparse region in the bladder base. Exogenous NO produced an elevation in cGMP in SUCs and SU-ICs. The distribution and proportion of these target cells varied between the dome, lateral wall and base. cGMP+ SU-ICs were present as a dense layer in the bladder base but were rarely seen in the lateral wall, which contained nNOS+ BUCs. No nNOS+ BUCs and cGMP+ SU-ICs were apparent in the dome. The degree of complexity in nNOS distribution and NO target cells is therefore greater than has previously been described and may reflect distinct physiological functions that have yet to be identified.







Similar content being viewed by others
References
Andersson KE (2002) Bladder activation: afferent mechanisms. Urology 59 (Suppl 1):43–50
Andersson KE, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986
Behrends S, Kempfert J, Mietens A, Koglin M, Scholz H, Middendorf R (2001) Developmental changes of nitric oxide-sensitive guanylyl cyclase expression in pulmonary arteries. Biochem Biophys Res Commun 283:883–887
Birder LA, Apodaca G, De Groat WC, Kanai AJ (1998) Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol 275:F226–F229
Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ (2001) Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A 98:13396–13401
Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ (2002) Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22:8063–8070
Burnstock G (2001) Purinergic signalling in the lower urinary tract. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology, vol 151/I. Purinergic and pyrimidinergic signalling I—molecular, nervous and urogenitary system function. Springer, Berlin Heidelberg New York, pp 89–127
Burton TJ, Elneil S, Nelson CP, Ferguson DR (2000) Activation of epithelial Na(+) channel activity in the rabbit urinary bladder by cAMP. Eur J Pharmacol 404:273–280
Burton TJ, Edwardson JM, Ingham J, Tempest HV, Ferguson DR (2002) Regulation of Na+ channel density at the apical surface of rabbit urinary bladder epithelium. Eur J Pharmacol 448:215–223
Chertin B, Rolle U, Solari V, Cascio S, Puri P (2004) The role of nitric oxide in bladder urothelial injury after bladder outlet obstruction. BJU Int 94:392–399
Cook HT, Bune AJ, Jansen AS, Taylor GM, Loi RK, Cattell V (1994) Cellular localization of inducible nitric oxide synthase in experimental endotoxic shock in the rat. Clin Sci (Lond) 87:179–186
de Vente J, Hopkins DA, Markerink-van Ittersum M, Emson PC, Schmidt HHHW, Steinbusch HWM (1998) Distribution of nitric oxide synthase and nitric oxide-receptive, cyclic GMP-producing structures in the rat brain. Neuroscience 87:207–241
Drake MJ, Harvey IJ, Gillespie JI (2003) Autonomous activity in the isolated guinea pig bladder. Exp Physiol 88:19–30
Ferguson DR (1999) Urothelial function. BJU Int 84:235–242
Ferguson DR, Twite BR (1975) The effect of diuretics on Na+−K+−ATPase and c-AMP levels in toad bladder epithelial cells. Naunyn Schmiedebergs Arch Pharmacol 287:111–116
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes-a possible sensory mechanism? J Physiol (Lond) 505:503–511
Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP (2004) Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology 63:24–31
Fujiwara M, Andersson K, Persson K (2000) Nitric oxide-induced cGMP accumulation in the mouse bladder is not related to smooth muscle relaxation. Eur J Pharmacol 401:241–250
Gillespie JI (2004a) The autonomous bladder: a view of the origin of bladder overactivity. BJU Int 93:478–483
Gillespie JI (2004b) Modulation of autonomous contractile activity in the isolated whole bladder of the guinea pig. BJU Int 93:393–400
Gillespie JI (2005) Inhibitory actions of calcitonin gene related peptide and capsaicin: evidence for local axonal reflexes in the bladder wall. BJU Int 95:149–156
Gillespie JI, Drake MJ (2004) The actions of sodium nitroprusside and the phosphodiesterase inhibitor dipyridimole on phasic activity in the isolated guinea pig bladder. BJU Int 93:851–858
Gillespie JI, Harvey IJ, Drake MJ (2003) Agonist and nerve induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol 88:343–357
Gillespie JI, Markerink-van Ittersum M, de Vente J (2004) cGMP generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int 94:1114–1124
Hawthorn MH, Chapple CR, Cock M, Chess-Williams R (2000) Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129:416–419
Johansson R, Pandita RK, Poljakovic M, Garcia-Pascual A, de Vente J, Persson K (2002) Activity and expression of nitric oxide synthase in the hypertrophied rat bladder and the effect of nitric oxide on bladder smooth muscle growth. J Urol 168:2689–2694
Lagou M, Drake MJ, Gillespie JI (2005) Volume-induced effects on the isolated bladder: a possible local reflex. BJU Int 94:1356–1365
Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML (2002) Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol 283:F242–F253
Lee HY, Bardini M, Burnstock G (2000) Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163:F2002–F2007
Lewis SA (2000) Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 278:FF867–FF874
Lewis SA, Wills NK (1983) Apical membrane permeability and kinetic properties of the sodium pump in rabbit urinary bladder. J Physiol (Lond) 341:169–184
Markert T, Vaandrager AB, Gambaryan S, Pohler D, Hausler C, Walter U, De Jonge HR, Jarchau T, Lohmann SM (1995) Endogenous expression of type II cGMP-dependent protein kinase mRNA and protein in rat intestine. Implications for cystic fibrosis transmembrane conductance regulator. J Clin Invest 96:822–830
McCloskey KD, Gurney AM (2002) Kit-positive cells in the guinea pig bladder. J Urol 168:832–836
Moon A (2002) Influence of nitric oxide signaling pathways on pre-contracted human detrusor smooth muscle in vitro. BJU Int 89:942–949
Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N (1999) Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol 162:2211–2216
Persson K, Igawa Y, Mattiasson A, Andersson KE (1992) Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol 107:178–184
Rong W, Spyer KM, Burnstock G (2002) Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol (Lond) 541:591–600
Smet PJ, Edyvane KA, Jonavicius J, Marshall VR (1996) Neuropeptides and neurotransmitter-synthesizing enzymes in intrinsic neurons of the human urinary bladder. J Neurocytol 25:112–124
Smet PJ, Jonavicius J, Marshall VR, de Vente J (1996) Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 71:337–348
Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ (2002) Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90:118–129
Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G (2002) Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13:830–846
Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21:5670–7567
Wiseman OJ, Fowler CJ, Landon DN (2003) The role of the human bladder lamina propria myofibroblast. BJU Int 91:89–93
Wu XR, Manabe M, Yu J, Sun TT (1990) Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem 265:19170–19179
Wu C, Sui GP, Fry CH (2004) Purinergic regulation of guinea pig suburothelial myofibroblasts.J Physiol (Lond) 559:231–243
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23
Zhou Y, Ling EA (1998) Co-localization of nitric oxide synthase and some neurotransmitters in the intramural ganglia of the guinea pig urinary bladder. J Comp Neurol 394:496–505
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gillespie, J.I., Markerink-van Ittersum, M. & de Vente, J. Expression of neuronal nitric oxide synthase (nNOS) and nitric-oxide-induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res 321, 341–351 (2005). https://doi.org/10.1007/s00441-005-1151-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-1151-3