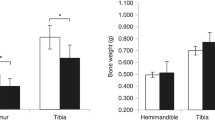
Abstract
Tooth eruption consists of the movement of teeth from the bony crypt in which they initiate their development to the occlusal plane in the oral cavity. Interactions between the tooth germ and its surrounding alveolar bone occur in order to offer spatial conditions for its development and eruption. This involves bone remodeling during which resoption is a key event. Bisphosphonates are a group of drugs that interfere with the resorption of mineralized tissues. With the purpose of investigating the effects of sodium alendronate (a potent bisphosphonate inhibitor of osteoclast activity) on alveolar bone during tooth development and eruption, we gave newborn rats daily doses of this drug for 4, 14, and 30 days. Samples of the maxillary alveolar process containing the tooth germs were processed for light, transmission, and scanning electron microscopy and were also submitted to tartrate-resistant acid phosphatase histochemistry and high-resolution colloidal-gold immunolabeling for osteopontin. Inhibition of osteoclast activity by sodium alendronate caused the absence of tooth eruption. The lack of alveolar bone remodeling resulted in primary bone with the presence of latent osteoclasts and abundant osteopontin at the interfibrillar regions. The developing bone trabeculae invaded the dental follicle and reached the molar tooth germs, provoking deformities in enamel surfaces. No root formation was observed. These findings suggested that alendronate effectively inhibited tooth eruption by interfering with the activation of osteoclasts, which remained in a latent stage.






Similar content being viewed by others
References
Arana-Chavez VE, Nanci A (2001) High-resolution immunocytochemistry of noncollagenous matrix proteins in rat mandibles processed with microwave irradiation. J Histochem Cytochem 49:1099–1110
Bonafe-Oliveira L, Faltin RM, Arana-Chavez VE (2003) Ultrastructural and histochemical examination of alveolar bone at the pressure areas of rat molars submitted to continuous orthodontic force. Eur J Oral Sci 111:410–416
Cahill DR, Marks SC Jr (1980) Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol 9:189–200
Greenblatt D (2005) Treatment of postmenopausal osteoporosis. Pharmacotherapy 25:574–584
Grier RL, Wise GE (1998) Inhibition of tooth eruption in the rat by a bisphosphonate. J Dent Res 77:8–15
Halasy-Nagy JM, Rodan GA, Reszka AA (2001) Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 29:553–559
Janones DS, Massa LF, Arana-Chavez VE (2005) Immunocytochemical examination of the presence of amelogenin during the root development of rat molars. Arch Oral Biol 50:527–532
Kitahara Y, Suda N, Kuroda T, Beck F, Hammond VE, Takano Y (2002) Disturbed tooth development in parathyroid hormone-related protein (PTHrP)-gene knockout mice. Bone 30:48–56
Kitahara Y, Suda N, Terashima T, Baba O, Mekaapiruk K, Hammond VE, Takano Y, Ohyama K (2004) Accelerated bone formation and increased osteoblast number contribute to the abnormal tooth germ development in parathyroid hormone-related protein knockout mice. Bone 35:1100–1106
Lerner UH (2000) Osteoclast formation and resorption. Matrix Biol 19:107–120
Lussier D, Huskey AG, Portenoy RK (2004) Adjuvant analgesics in cancer pain management. Oncologist 9:571–591
Maasalu K, Haviko T, Martson A (2003) Treatment of children with osteogenesis imperfecta in Estonia. Acta Paediatr 92:452–455
Marks SC Jr (1973) Pathogenesis of osteopetrosis in the IA rat: reduced bone resorption due to reduced osteoclast function. Am J Anat 138:165–189
Marks SC Jr, Schroeder HE (1996) Tooth eruption: theories and facts. Anat Rec 245:374–393
Massa LF, Arana-Chavez VE (2000) Ultrastructural preservation of rat embryonic dental tissues after rapid fixation and dehydratation under microwave irradiation. Eur J Oral Sci 108:74–77
Massa LF, Bradaschia-Correa V, Arana-Chavez VE (2006) Immunocytochemical study of amelogenin deposition during the early odontogenesis of molars in alendronate-treated newborn rats. J Histochem Cytochem 54:713–725
McClung M (2003) Use of highly potent bisphosphonates in the treatment of osteoporosis. Curr Osteoporos Rep 1:116–122
McKee MD, Nanci A (1996) Osteopontin: an interfacial extracellular matrix protein in mineralized tissues. Connect Tissue Res 35:197–205
Muller S, Migianu E, Lecouvey M, Kraemer M, Oudar O (2005) Alendronate inhibits proliferation and invasion of human epidermoid carcinoma cells in vitro. Anticancer Res 25:2655–2660
Nanci A (1999) Content and distribution of noncollagenous matrix proteins in bone and cementum: relationship to speed of formation and collagen packing density. J Struct Biol 126:256–269
Nanci A, Zalzal S, Gotoh Y, McKee MD (1996) Ultrastructural characterization and immunolocalization of osteopontin in rat calvarial osteoblast primary cultures. Microsc Res Tech 33:214–231
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88:2961–2978
Santini D, Gentilucci UV, Vicenzi B, Picardi A, Vasaturo F, La Cesa A, Onori N, Scarpa S, Tonini G (2003) The antineoplastic role of bisphosphonates: from basic research to clinical evidence. Ann Oncol 14:1468–1476
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action—alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88:2095–2105
Srivastava T, Alon US (2003) The role of bisphosphonates in diseases of childhood. Eur J Pediatr 162:735–751
Strong RM (1925) The order, time, and rate of ossification of the albino rat (Mus novergicus albinos) skeleton. Am J Anat 36:313–355
van Beek ER, Cohen LH, Leroy IM, Ebetino FH, Löwik CWGM, Papapoulos SE (2003) Differentiating the mechanisms of resorptive action of nitrogen containing bisphosphonates. Bone 33:805–811
Vasikaran SD (2001) Bisphosphonates: an overview with special reference to alendronate. Ann Clin Biochem 38:608–623
Ward K, Cowell CT, Little DG (2005) Quantification of metaphyseal modeling in children treated with bisphosphonates. Bone 36:999–1002
Wise GE, Frazier-Bowers S, D’Souza RN (2002) Cellular, molecular and genetic determinants of tooth eruption. Crit Rev Oral Biol Med 13:323–334
Zeichner-David M, Oishi K, Su Z, Chen IS, Arzate H, Bringas P (2003) Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev Dyn 228:651–663
Acknowledgements
The authors thank Dr. Antonio Nanci (University of Montreal, Canada) for supplying the anti-rat OPN antibody, and Dr. Lucienne Bonafe-Oliveira for her help during the histochemical procedures. Technical assistance by Mrs. Fernanda Barrence and Mr. Gerson B. Silva (in memoriam), ultrathin sectioning by Mr. Gaspar F. de Lima, and production of electron micrographs by Mr. Edson Oliveira are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from Fapesp (04/05831-9 and 06/60094-5) and CNPq (Brazil).
Rights and permissions
About this article
Cite this article
Bradaschia-Correa, V., Massa, L.F. & Arana-Chavez, V.E. Effects of alendronate on tooth eruption and molar root formation in young growing rats. Cell Tissue Res 330, 475–485 (2007). https://doi.org/10.1007/s00441-007-0499-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0499-y