Abstract
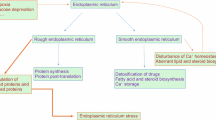
Ovarian surface epithelium (OSE) forms a single layer of mostly cuboidal cells on surface of mammalian ovaries that is inherently exposed to cell stress evoked by tissue damage every ovulation and declines morphologically after menopause. Endoplasmic reticulum (ER) is a principal cell organelle involved in proteosynthesis, but also integrating various stress signals. ER stress evokes a conserved signaling pathway, the unfolded protein response (UPR), leading to cell death or adaptation to stress conditions. In this work, we document that mouse OSE suffers from ER stress during replicative senescence in vitro, develops abnormalities in ER and initiates UPR. Attenuation of ER stress in senescent OSE by tauroursodeoxycholic acid (TUDCA) reconditions ER architecture and leads to delayed onset of senescence. In summary, we show for the first time a mutual molecular link between ER stress response and replicative senescence leading to phenotypic changes of non-malignant ovarian surface epithelium.




Similar content being viewed by others
References
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22:255–288
Cho JG, Lee JH, Hong SH, Lee HN, Kim CM, Kim SY, Yoon KJ, Oh BJ, Kim JH, Jung SY, Asahara T, Kwon SM, Park SG (2015) Tauroursodeoxycholic acid, a bile acid, promotes blood vessel repair by recruiting vasculogenic progenitor cells. Stem Cells 33:792–805
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol-Mech 5:99–118
Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 10:1511–1518
Fathalla MF (2013) Incessant ovulation and ovarian cancer—a hypothesis re-visited. Facts Views Vis Obgyn 5:292–297
Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, Kim HW, Pasha Z, Wen Z, Rao F, Modi RM, Yu X, Ashraf M (2014) Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 32:462–472
Flesken-Nikitin A, Hwang C-I, Cheng C-Y, Michurina TV, Enikolopov G, Nikitin AY (2013) Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 495:241
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Horak P, Tomasich E, Vanhara P, Kratochvilova K, Anees M, Marhold M, Lemberger CE, Gerschpacher M, Horvat R, Sibilia M, Pils D, Krainer M (2014) TUSC3 loss alters the ER stress response and accelerates prostate Cancer growth in vivo. Sci Rep 4:3739
Huang N, Yu Y, Qiao J (2017) Dual role for the unfolded protein response in the ovary: adaption and apoptosis. Protein Cell 8:14–24
Kido M, Shibuya M (1998) Isolation and characterization of mouse ovarian surface epithelial cell lines. Pathol Res Pract 194:725–730
King SM, Quartuccio SM, Vanderhyden BC, Burdette JE (2013) Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require Akt upregulation, DNA damage and epithelial-stromal interaction. Carcinogenesis 34:1125–1133
Kratochvilova K, Horak P, Esner M, Soucek K, Pils D, Anees M, Tomasich E, Drafi F, Jurtikova V, Hampl A, Krainer M, Vanhara P (2015) Tumor suppressor candidate 3 (TUSC3) prevents the epithelial-to-mesenchymal transition and inhibits tumor growth by modulating the endoplasmic reticulum stress response in ovarian cancer cells. Int J Cancer 137:1330–1340
Kratochvílová K, Moráň L, Paďourová S, Stejskal S, Tesařová L, Šimara P, Hampl A, Koutná I, Vaňhara P (2016) The role of the endoplasmic reticulum stress in stemness, pluripotency and development. Eur J Cell Biol
Kurman RJ, Shih Ie M (2016) The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol 186:733–747
Kyathanahalli C, Organ K, Moreci RS, Anamthathmakula P, Hassan SS, Caritis SN, Jeyasuria P, Condon JC (2015) Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and-7-dependent. Proc Natl Acad Sci U S A 112:14090–14095
Laszczynska M, Brodowska A, Starczewski A, Masiuk M, Brodowski J (2008) Human postmenopausal ovary—hormonally inactive fibrous connective tissue or more? Histol Histopathol 23:219–226
Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA (2010) Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia 12:317–325
McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ (2004) Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18:355–357
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow PK, Norris FH, Helvering L, Morrison P, Rosteck P, Lai M, Barrett JC, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar D, Wiseman R, Kamb A, Skolnick MH (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71
Okamura H, Katabuchi H, Nitta M, Ohtake H (2006) Structural changes and cell properties of human ovarian surface epithelium in ovarian pathophysiology. Microsc Res Tech 69:469–481
Pils D, Horak P, Vanhara P, Anees M, Petz M, Alfanz A, Gugerell A, Wittinger M, Gleiss A, Auner V, Tong D, Zeillinger R, Braicu EI, Sehouli J, Krainer M (2013) Methylation status of TUSC3 is a prognostic factor in ovarian cancer. Cancer 119:946–954
Pluquet O, Pourtier A, Abbadie C (2015) The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol 308:C415–C425
Uppala JK, Gani AR, Ramaiah KVA (2017) Chemical chaperone, TUDCA unlike PBA, mitigates protein aggregation efficiently and resists ER and non-ER stress induced HepG2 cell death. Sci Rep 7:3831
Urzua U, Ampuero S, Roby KF, Owens GA, Munroe DJ (2016) Dysregulation of mitotic machinery genes precedes genome instability during spontaneous pre-malignant transformation of mouse ovarian surface epithelial cells. BMC Genomics 17:728
Wang M, Kaufman RJ (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 14:581–597
Xie YX, He YG, Cai ZL, Cai JH, Xi MY, Zhang YD, Xi JK (2016) Tauroursodeoxycholic acid inhibits endoplasmic reticulum stress, blocks mitochondrial permeability transition pore opening, and suppresses reperfusion injury through GSK-3 beta in cardiac H9c2 cells. Am J Transl Res 8:4586–4597
Yeung BHY, Kwan BWY, He QY, Lee AS, Liu J, Wong AST (2008) Glucose-regulated protein 78 as a novel effector of BRCA1 for inhibiting stress-induced apoptosis. Oncogene 27:6782–6789
Yoon YM, Lee JH, Yun SP, Han YS, Yun CW, Lee HJ, Noh H, Lee SJ, Han HJ, Lee SH (2016) Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci Rep 6:39838
Acknowledgements
We acknowledge the core facility CELLIM of CEITEC supported by the Czech-BioImaging large RI project (project no. LM2015062 funded by MEYS CR) for their support with obtaining scientific data presented in this paper.
Funding
This work was supported by the Grant Agency of Masaryk University (project no. MUNI/A/1298/2017) and European Regional Development Fund (Center for Analysis and Modeling of Tissues and Organs, CZ.1.07/2.3.00/20.0185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Animal experiments were supervised by the local ethical committee of the Faculty of Medicine, Masaryk University and performed by certified individuals (KV, PV, and LM). This article does not contain any studies with human participants performed by any of the authors.
Competing interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig. S1
UPR is elicited in intact ovarian explants cultured under ER stress promoting conditions. Dissected ovarian explants were left untreated (a-c) or cultured for 24 h in presence of 0.5 μM tunicamycin (d-f) and processed for immunohistochemistry of HSPA5 and DDIT3. Staining for KRT8 validates intactness of ovarian surface epithelial layer. Scale bars indicate 200 μm (a-c) or 100 μm (d-f) (PNG 2964 kb)
Rights and permissions
About this article
Cite this article
Vašíčková, K., Moráň, L., Gurín, D. et al. Alleviation of endoplasmic reticulum stress by tauroursodeoxycholic acid delays senescence of mouse ovarian surface epithelium. Cell Tissue Res 374, 643–652 (2018). https://doi.org/10.1007/s00441-018-2888-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2888-9