Abstract
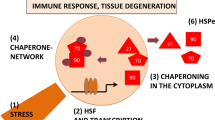
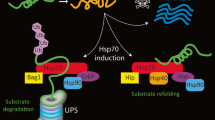
Stress, a common phenomenon in today’s society, is suspected of playing a role in the development of disease. Stressors of various types, psychological, physical, and biological, abound. They occur in the working and social environments, in air, soil, water, food, and medicines. Stressors impact on cells directly or indirectly, cause protein denaturation, and elicit a stress response. This is mediated by stress (heat-shock) genes and proteins, among which are those named molecular chaperones because they assist other proteins to achieve and maintain a functional shape (the native configuration), and to recover it when partially lost due to stress. Denatured proteins tend to aggregate and precipitate. The same occurs with abnormal proteins due to mutations, or to failure of post-transcriptional or post-translational mechanisms. These abnormal proteins need the help of molecular chaperones as much as denatured molecules do, especially during stress. A cell with normal antistress mechanisms, including a complete and functional set of chaperones, may be able to withstand stress if its intensity is not beyond that which will cause irreversible protein damage. There is a certain threshold that normal cells have above which they cannot cope with stress. A cell with an abnormal protein that has an intrinsic tendency to misfold and aggregate is more vulnerable to stress than normal counterparts. Furthermore, these abnormal proteins may precipitate even in the absence of stress and cause diseases named proteinopathies. It is possible that stress contributes to the pathogenesis of proteinopathies by promoting protein aggregation, even in cells that possess a normal chaperoning system. Examples of proteinopathies are age-related degenerative disorders with protein deposits in various tissues, most importantly in the brain where the deposits are associated with neuronal degeneration. It is conceivable that stress enhances the progression of these diseases by facilitating protein unfolding and misfolding, which lead to aggregation and deposition. A number of reports in the last few years have described research aimed at elucidating the role of heatshock proteins, molecular chaperones in particular, in the pathogenesis of neurodegenerative disorders. The findings begin to shed light on the molecular mechanism of protein aggregation and deposition, and of the ensuing cell death. The results also begin to elucidate the role of molecular chaperones in pathogenesis. This is a fascinating area of research with great clinical implications. Although there are already several experimental models for the study of proteinopathies, others should be developed using organisms that are better known now than only a few years ago and that offer unique advantages. Use of these systems and of information available in databases from genome sequencing efforts should boost research in this field. It should be possible in the not-too-distant future to develop therapeutic and preventive means for proteinopathies based on the use of heat-shock protein and molecular chaperone genes and proteins.
Similar content being viewed by others
References
Macario AJL. Heat-shock proteins and molecular chaperones: implications for pathogenesis, diagnostics, and therapeutics. Int J Clin Lab Res 1995; 25:59.
Macario AJL, Conway de Macario E. The archaeal molecular chaperone machine: peculiarities and paradoxes. Genetics 1999; 152:1277.
Macario AJL, Lange M, Ahring BK, Conway de Macario E. Stress genes and proteins in the Archaea. Microbiol Mol Biol Rev 1999; 63:923.
Conway de Macario E, Macario AJL. Molecular biology of stress genes in methanogens: potential for bioreactor technology. Adv Biochem Eng Biotechnol. In press.
Macario AJL, Conway de Macario E. Molecular chaperones and age-related degenerative disorders. In: Mattson MP, ed. Interorganellar signaling in age-related disease. Amsterdam, The Netherlands: Elsevier Science Publishers. In press.
Macario AJL, Conway de Macario E. Heat-shock response, overview. In: Fink G, ed. The encyclopedia of stress, vol. 2. San Diego, California, USA: Academic Press. 2000:429.
Macario AJL, Conway de Macario E. Chaperone proteins. In: Fink G, ed. The encyclopedia of stress, vol. 1. San Diego, California, USA: Academic Press. 2000:350.
Hartl FU, Martin J. Molecular chaperones in cellular protein folding. Curr Biol 1995; 5:92.
Jensen RE, Johnson AE. Protein translocation: is Hsp70 pulling my chain. Curr Biol 1999; 9:R779.
Leroux M, Fändrich M, Klunker D, Siegers K, Lupas AN, Brown JR, Schiebel E, Dobson CM, Hartl FU. MtGimC, a novel archaeal chaperone related to the eukaryotic chaperonin cofactor GimC/prefoldin. EMBO J 1999; 18:6730
Tansey WP. How cells use proteolysis to control their growth. Mol Med 1999; 5:773.
Wickner S, Maurizi MR, Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science 1999; 286:1888.
Frazier LD. Coping with disease-related stressors in Parkinson’s disease. Gerontologist 2000; 40:53.
Hänninen A-L, Simola M, Makarow M. The cytoplasmic chaperone Hsp104 is required for conformational repair of heat-denatured proteins in yeast endoplasmic reticulum. Mol Biol Cell 1999; 10:3623.
Wang X-Y, Chen X, Oh H-J, Repasky E, Kazim L, Subject J. Characterization of native interaction of hsp110 with hsp25 and hsc70. FEBS Lett 2000; 464:98.
Caplan AJ. Hsp90’s secrets unfold: new insights from structural and functional studies. Trends Cell Biol 1999; 9:262.
Kanelakis KC, Morishima Y, Dittmar KD, Galigniana MD, Takayama S, Reed JC, Pratt WB. Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J Biol Chem 1999; 274:34134.
Russell LC, Whitt SR, Chen M-S, Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem 1999; 274:20060.
Ihara Y, Cohen-Doyle MF, Saito Y, Williams DB. Calnexin discriminates between protein conformational states and functions as molecular chaperone in vitro. Mol Cell 1999; 4:331.
Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J 1999; 344:281.
Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J 1999; 18:6718.
Zapun A, Jakob CA, Thomas DY, Bergeron JJM. Protein folding in a specialized compartment: the endoplasmic reticulum. Structure 1999; 7:R173.
King C, Eisenberg E, Greene LE. Interaction between Hsc70 and DnaJ homologues: relationship between Hsc70 polymerization and ATPase activity. Biochemistry 1999; 38:12452.
Liu F-H, Wu S-J, Hu S-M, Hsiao C-D, Wang C. Specific interaction of the 70-kDa heat shock cognate protein with the tetratricopeptide repeats. J Biol Chem 1999; 274:34425.
Nollen EAA, Brunsting JF, Song J, Kampinga HH, Morimoto RI. Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol Cell Biol 2000; 20:1083.
Stuhlmeier KM. Activation and regulation of Hsp32 and Hsp70. Eur J Biochem 2000; 267:1161.
Welihinda AA, Tirasophon W, Kaufman RJ. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr 1999; 7:293.
Llorca O, McCormack EA, Hynes G, Grantham J, Cordell J, Carrascosa JL, Willison KR, Fernandez JJ, Valpuesta JM. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 1999; 402:693.
Yokota S-I, Yanagi H, Yura T, Kubota H. Upregulation of cytosolic chaperonin CCT subunits during recovery from chemical stress that causes accumulation of unfolded proteins. Eur J Biochem 2000; 267:1658.
Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. EMBO J 1999; 18:2040.
Sanchez GI, Carucci DJ, Sacci J, Resau JH, Rogers WO, Kumar N, Hoffman SL.Plasmodium yoelii: cloning and characterization of the gene encoding for the mitochondrial heat shock protein 60. Exp Parasitol 1999; 93:181.
Chen M-S, Roti RJ, Laszlo A. Hsc40, a new member of the hsp40 family, exhibits similar expression profile to that of hsc70 in mammalian cells. Gene 1999; 238:333.
Kelley WL. Molecular chaperones: how J domains turn on Hsp70s. Curr Biol 1999; 9:R305.
Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J 1999; 18:6744.
Perng MD, Muchowski PJ, van Den IJssel P, Wu GJS, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in αB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem 1999; 274:33235.
Bao W-G, Huo K-K, Li Y-Y, Fukuhara H. Protein disulphide isomerase genes ofKluyveromyces lactis. Yeast 2000; 16:329.
Bechtold DA, Brown IR. Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Mol Brain Res 2000; 75:309.
Herdegen T, Fischer G, Gold BG. Immunophilin ligands as a novel treatment for neurological disorders. Trends Pharmacol Sci 2000; 21:3.
Reddy GB, Das KP, Petrash JM, Surewickz WK. Temperature-dependent chaperone activity and structural properties of human αA- and αB-crystallins. J Biol Chem 2000; 275:4565.
Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Gen Dev 1999; 13:1211.
Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell 1999; 99:691.
Piccinini M, Tazartes O, Mostert M, Musso A, DeMarchi M, Rinaudo MT. Structural and functional characterization of 20S and 26S proteasomes from bovine brain. Mol Brain Res 2000; 76:103.
Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem 1999; 274:6875.
Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci 2000; 25:95.
Bogdanov M, Dowhan W. Lipid-assisted protein folding. J Biol Chem 1999; 274:36827.
Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol 2000; 10:73
Landry J, Chretien P, Lanbert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human Hsp27 gene in rodent cells. J Cell Biol 1989; 109:7.
Lavoie JN, Gigras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cell confers resistance to heat shock. Hsp27 stabilization of the microfilament organization. J Biol Chem 1993; 286:3420.
Martin JL, Hickey E, Weber LA, Dillman WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene Expr. 1999; 7:349.
Nakamura S, Kawamoto Y, Nakano S, Ikemoto A, Akiguchi I, Kimura J. Cyclin-dependent kinase 5 in Lewy body-like inclusions in anterior horn cells of a patient with sporadic amyotrophic lateral sclerosis. Neurology 1997; 48:267.
Davis RL, Holohan PD, Shrimpton AE, Tatum AH, Daucher J, Collins GH, Todd R., Bradshaw C, Kent P, Feiglin D, Rosenbaum A, Yerby MS, Shaw C-M, Lacbawan F, Lawrence DA. Familial encephalopathy with neuroserpin inclusion bodies. Am J Pathol 1999; 155:1901.
Fergusson J, Landon M, Lowe J, Ward L, van FW Leeuwen, Mayer RJ. Neurofibrillary tangles in progressive supranuclear palsy brains exhibit immunoreactivity to frameshift mutant ubiquitin-B protein. Neurosci Lett 2000; 279:69.
Furlong RA, Narain Y, Rankin J, Wyttenbach A, Rubinsztein DC. α-synuclein overexpression promotes aggregation of mutant huntingtin. Biochem J 2000; 346:577.
Schwab C, McGeer PL. Aβ42-carboxy-terminal-like immunoreactivity is associated with intracellular neurofibrillary tangles and Pick bodies. Exp Neurol 2000; 161:527.
Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1999; 1:479.
Yang Y, Turner RS, Gaut JR. The chaperone BiP/GRP78 binds to amyloid precursor protein and decrease Aβ40 and Aβ42 secretion. J Biol Chem 1998; 273:25552.
Lu P-J, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999; 399:784.
Mandelkow E. The tangled tale of tau. Nature 1999; 402:588.
Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet 1998; 19:148.
Chai Y, Koppenhafer SL, Bonini NM, Paulson HL. Analysis of the role of heat shock (Hsp) molecular chaperones in polyglutamine disease. J Neurosci 1999; 19:10388.
Stenoien DL, Cummings CJ, Adams HP, Mancini MG, Patel K, DeMartino GN, Marcelli M, Weigel NL, Mancini MA. Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone. Hum Mol Genet 1999; 8:731.
Warrick JM, Chan HYE, Gray-Board GL, Chai YY, Paulson HL, Bonini N. Suppression of polyglutamine-mediated neurodegeration inDrosophila by the molecular chaperone HSP70. Nat Genet 1999; 23:425.
Kazemi-Esfarjani P, Benzer S. Genetic suppression of polyglutamine toxicity inDrosophila. Science 2000; 287:1837.
Lamb JR, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci 1995; 20:257.
Groves MR, Barford D. Topological characteristics of helical repeat proteins. Curr Op in Struct Biol 1999; 9:983.
Wyttenbach A, Carmichael J, Swartz J, Furlong RA, Narain Y, Rankin J, Rubinsztein DC. Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington’s disease. Proc Natl Acad Sci USA 2000; 97:2898.
Krobitsch S, Linquist S. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci USA 2000; 97:1589.
Chernoff YO, Newman GP, Kumar J, Allen K, Zink AD. Evidence for a protein mutator in yeast: role of the Hsp70-related chaperone Ssb in formation, stability, and toxicity of the [PSI] prion. Mol Cell Biol 1999; 19:8103.
Yan SD, Roher A, Schmidt AM, Stern DM. Cellular cofactors for amyloid β-peptide-induced cell stress. Am J Pathol 1999; 155:1403.
Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Woloseker H, Barañano DE, Doré S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1999; 1:152.
Doré S, Sampei K, Goto S, Alkayed NJ, Guastella D, Balckshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med 1999; 5:656.
Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 1998; 12:3788
Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol 2000; 156:857.
Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576.
Woese CR. A manifesto for microbial genomics. Curr Biol 1998; 8:R781.
Yutani K, Takayama G, Goda S, Yamagata Y, Maki S, Namba K, Tsunasawa S, Ogasahara K. The process of amyloid-like fibril formation by methionine aminopeptidase from a hyperthermophile,Pyrococcus furiosus. Biochemistry 2000; 39:2769.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macario, A.J.L., Conway de Macario, E. Stress and molecular chaperones in disease. Int J Clin Lab Res 30, 49–66 (2000). https://doi.org/10.1007/s005990070016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s005990070016