Abstract
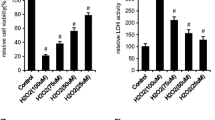
Increasing evidence suggests a role for oxidative stress in age-related decrease in osteoblast number and function leading to the development of osteoporosis. This study was undertaken to investigate whether ghrelin, previously reported to stimulate osteoblast proliferation, counteracts tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in MC3T3-E1 osteoblastic cells as well as to characterize the ghrelin receptor (GHS-R) involved in such activity. Pretreatment with ghrelin (10−7–10−11 M) significantly increased viability and reduced apoptosis of MC3T3-E1 cells cultured with t-BHP (250 μM) for three hours at the low concentration of 10−9 M as shown by MTT assay and Hoechst-33258 staining. Furthermore, ghrelin prevented t-BHP-induced osteoblastic dysfunction and changes in the cytoskeleton organization evidenced by the staining of the actin fibers with Phalloidin-FITC by reducing reactive oxygen species generation. The GHS-R type 1a agonist, EP1572 (10−7–10−11 M), had no effect against t-BHP-induced cytotoxicity and pretreatment with the selective GHS-R1a antagonist, d-Lys3-GHRP-6 (10−7 M), failed to remove ghrelin (10−9 M)-protective effects against oxidative injury, indicating that GHS-R1a is not involved in such ghrelin activity. Accordingly, unacylated ghrelin (DAG), not binding GHS-R1a, displays the same protective actions of ghrelin against t-BHP-induced cytotoxicity. Preliminary observations indicate that ghrelin increased the trimethylation of lys4 on histones H3, a known epigenetic mark activator, which may regulate the expression of some genes limiting oxidative damage. In conclusion, our data demonstrate that ghrelin and DAG promote survival of MC3T3-E1 cell exposed to t-BHP-induced oxidative damage. Such effect is independent of GHS-R1a and is likely mediated by a common ghrelin/DAG binding site.










Similar content being viewed by others
References
Aleshin S, Reiser G (2013) Role of the peroxisome proliferator-activated receptors (PPAR)-α, β/δ and γ triad in regulation of reactive oxygen species signaling in brain. Biol Chem. doi:10.1515/hsz-2013-0215 (Epub ahead of print)
Almeida M (2012) Aging mechanisms in bone. Bonekey Rep1
Almeida M, Han L, Martin-Millan M et al (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Altman SA, Zastawny TH, Randers L et al (1994) tert-Butyl hydroperoxide-mediated DNA base damage in cultured mammalian cells. Mutat Res 306:35–44
Bai XC, Lu D, Bai J et al (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314:197–207
Baldanzi G, Filigheddu N, Cutrupi S et al (2002) Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol 159:1029–1037
Banfi G, Iorio EL, Corsi MM (2008) Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med 46:1550–1555
Basu S, Michaëlsson K, Olofsson H et al (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288:275–279
Bednarek MA, Feighner SD, Pong SS et al (2000) Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43:4370–4376
Broglio F, Boutignon F, Benso A et al (2002) EP1572: a novel peptido-mimetic GH secretagogue with potent and selective GH-releasing activity in man. J Endocrinol Invest 25:26–28
Carreira MC, Camiña JP, Smith RG, Casanueva FF (2004) Agonist-specific coupling of growth hormone secretagogue receptor type 1a to different intracellular signaling systems. Role of adenosine. Neuroendocrinology 79:13–25
Carreira MC, Camiña JP, Díaz-Rodríguez E et al (2006) Adenosine does not bind to the growth hormone secretagogue receptor type-1a (GHS-R1a). J Endocrinol 191:147–157
Cocchi D, Maccarinelli G, Sibilia V et al (2005) GH-releasing peptides and bone. J Endocrinol Invest 28(8 Suppl):11–14
Cos P, Hermans N, Calomme M et al (2003) Comparative study of eight well-known polyphenolic antioxidants. J Pharm Pharmacol 55:1291–1297
Costa JL, Naot D, Lin JM et al (2011) Ghrelin is an osteoblast mitogen and increases osteoclastic bone resorption in vitro. Int J Pept 2011:605193. doi:10.1155/2011/605193
Delhanty PJ, van der Eerden BC, van der Velde M et al (2006) Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol 188:37–47
Delhanty PJ, van der Eerden BC, van Leeuwen JP (2013) Ghrelin and bone. Biofactors. doi:10.1002/biof.1120 (Epub ahead of print)
El Eter E, Al Tuwaijiri A, Hagar H, Arafa M (2007) In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol 22:1791–1799
Fukushima N, Hanada R, Teranishi H et al (2005) Ghrelin directly regulates bone formation. J Bone Miner Res 20:790–798
Ghigo E, Broglio F, Arvat E et al (2005) Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol 62:1–17
Granata R, Settanni F, Biancone L et al (2007) Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology 148:512–529
Haidara K, Marion M, Gascon-Barré M et al (2008) Implication of caspases and subcellular compartments in tert-butyl hydroperoxide induced apoptosis. Toxicol Appl Pharmacol 229:65–76
Holmes E, Davies I, Lowe G, Ranganath LR (2009) Circulating ghrelin exists in both lipoprotein bound and free forms. Ann Clin Biochem 46:514–516
Hosoda H, Kojima M, Matsuo H, Kangawa K (2000) Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279:909–991
Huang Y, Min S, Lui Y et al (2012) Global mapping of H3K4me3 and H3K27me3 reveals chromatin state-based regulation of human monocyte-derived dendritic cells in different environments. Gene Immun 13:311–320. doi:10.1038/gene.87
Işeri SO, Sener G, Saglam B et al (2008) Ghrelin alleviates biliary obstruction-induced chronic hepatic injury in rats. Regul Pept 146:73–79
Kawczynska-Drozdz A, Olszanecki R, Jawien J et al (2006) Ghrelin inhibits vascular superoxide production in spontaneously hypertensive rats. Am J Hypertens 19(7):764–767
Kim SW, Her SJ, Park SJ et al (2005) Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone 37:359–369
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85:495–522
Lear PV, Iglesias MJ, Feijóo-Bandín S et al (2010) Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology 151:3286–3298
Liang QH, Liu Y, Wu SS et al (2013) Ghrelin inhibits the apoptosis of MC3T3-E1 cells through ERK and AKT signaling pathway. Toxicol Appl Pharmacol 272:591–597
Maccarinelli G, Sibilia V, Torsello A et al (2005) Ghrelin regulates proliferation and differentiation of osteoblastic cells. J Endocrinol 184:249–256
Maggio D, Barabani M, Pierandrei M et al (2003) Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab 88:1523–1527
Malagón MM, Luque RM, Ruiz-Guerrero E et al (2003) Intracellular signaling mechanisms mediating ghrelin-stimulated growth hormone release in somatotropes. Endocrinology 144:5372–5380
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300
Manolagas SC, Parfitt AM (2010) What old means to bone. Trends Endocrinol Metab 6:369–374
Muccioli G, Baragli A, Granata R et al (2007) Heterogeneity of ghrelin/growth hormone secretagogue receptors. Toward the understanding of the molecular identity of novel ghrelin/GHS receptors. Neuroendocrinology 86:147–164
Muller FL, Lustgarten MS, Jang Y et al (2007) Trends in oxidative aging theories. Free Radic Biol Med 43:477–503
Muthusami S, Ramachandran I, Muthusamy B et al (2005) Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta 360:81–86
Nikolopoulos D, Theocharis S, Kouraklis G (2010) Ghrelin, another factor affecting bone metabolism. Med Sci Monit 16:147–162
Nojiri H, Saita Y, Morikawa D et al (2011) Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res 26:2682–2694
Obay BD, Taşdemir E, Tümer C et al (2008) Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides 29:448–455
Ozgocmen S, Kaya H, Fadillioglu E et al (2007) Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem 295:45–52
Patterson M, Murphy KG, le Roux CW et al (2005) Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 90:2205–2211
Seeman E (2003) Invited Review: pathogenesis of osteoporosis. J Appl Physiol 95:2142–2151
Seim I, Josh P, Cunningham P et al (2011) Ghrelin axis genes, peptides and receptors: recent findings and future challenges. Mol Cell Endocrinol 340:3–9
Sestili P, Guidarelli A, Dachà M, Cantoni O (1998) Quercetin prevents DNA single strand breakage and cytotoxicity caused by tert-butylhydroperoxide: free radical scavenging versus iron chelating mechanism. Free Radic Biol Med 25:196–200
Sibilia V, Cocchi D, Pagani F et al (1999) Hexarelin, a growth hormone-releasing peptide, counteracts bone loss in gonadectomized male rats. Growth Horm IGF Res 9:219–227
Sibilia V, Cocchi D, Villa I et al (2002) Bone effects of hexarelin, a GH-releasing peptide, in female rats: influence of estrogen milieu. Eur J Endocrinol 146:855–862
Sibilia V, Muccioli G, Deghenghi R et al (2006) Evidence for a role of the GHS-R1a receptors in ghrelin inhibition of gastric acid secretion in the rat. J Neuroendocrinol 18:122–128
Sibilia V, Pagani F, Mrak E et al (2012) Pharmacological characterization of the ghrelin receptor mediating its inhibitory action on inflammatory pain in rats. Amino Acids 43:1751–1759
Svensson J, Lall S, Dickson SL et al (2000) The GH secretagogues ipamorelin and GH-releasing peptide-6 increase bone mineral content in adult female rats. J Endocrinol 165:569–577
Thompson NM, Gill DA, Davies R et al (2004) Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145:234–242
Tong XX, Wu D, Wang X et al (2012) Ghrelin protects against cobalt chloride-induced hypoxic injury in cardiac H9c2 cells by inhibiting oxidative stress and inducing autophagy. Peptides 38:217–227
van der Velde M, Delhanty P, van der Eerden B et al (2008) Ghrelin and bone. Vitam Horm 77:239–258
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol Med 27:612–616
Yang J, Brown MS, Liang G et al (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132:387–396
Zhang Q, Huang WD, Lv XY, Yang YM (2011) Ghrelin protects H9c2 cells from hydrogen peroxide-induced apoptosis through NF-κB and mitochondria-mediated signaling. Eur J Pharmacol 654:142–149
Acknowledgments
This work was supported by funds from FIRST 2009 to VS and from Project ASI DC-DTE-2011-033 for biomedical and biotechnological studies to FC.
Conflict of interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dieci, E., Casati, L., Pagani, F. et al. Acylated and unacylated ghrelin protect MC3T3-E1 cells against tert-butyl hydroperoxide-induced oxidative injury: pharmacological characterization of ghrelin receptor and possible epigenetic involvement. Amino Acids 46, 1715–1725 (2014). https://doi.org/10.1007/s00726-014-1734-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1734-y