Abstract
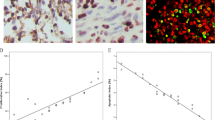
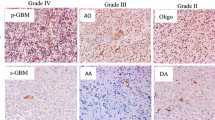
Accumulating evidence suggests that tissue hypoxia and apoptosis play important roles in the malignant progression of brain tumors. We investigated the relationship of 14-3-3zeta (an apoptosis-related protein), HIF-1α, and VEGF immunohistochemistry, and evaluated the prognostic value of their expression in human brain gliomas. A semiquantitative analysis of the immunoreactivity scores (IRSs) of the 14-3-3zeta, HIF-1α, and VEGF proteins was performed in 27 patients with various grades of gliomas. The IRS of 14-3-3zeta increased with tumor grade, with grade IV gliomas having the highest score (P < 0.05). Similar results were found for the IRSs of HIF-1α and VEGF (P < 0.05). A significant positive correlation was found between the IRSs of 14-3-3zeta and HIF-1α, 14-3-3zeta and VEGF, and HIF-1α and VEGF (P < 0.001 for all). The survival time of HIF-1α in grade III and grade IV glioma patients with low IRSs (0–6) was significantly longer than that in such glioma patients with high IRSs (8–12) (P < 0.05). These data indicate that 14-3-3zeta, HIF-1α, and VEGF are involved in the same cascade of the malignant progression of gliomas. Further studies will elucidate their detailed role in the malignant progression of glioma, and will contribute to the development of a new treatment strategy for this lethal disease.





Similar content being viewed by others
References
Louis DN (2006) Molecular pathology of malignant gliomas. Annu Rev Pathol Mech Dis 1:97–117
Louis DN, Ohgaki H, Wiestler OD (2007) WHO classification of tumors of the central nervous system, vol 1, 4th edn. IARC, Lyon
Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109:93–108
Rajendran JG, Mankoff DA, O’Sullivan F et al (2004) Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10:2245–2252
Brat DJ, Castellano-Sanchez AA, Hunter SB et al (2004) Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 64:920–927
Fischer I, Gagner JP, Law M et al (2005) Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 15:297–310
Tsang RW, Fyles AW, Milosevic M et al (2000) Interrelationship of proliferation and hypoxia in carcinoma of the cervix. Int J Radiat Oncol Biol Phys 46:95–99
Slomiany MG, Rosenzweig SA (2006) Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther 318:666–675
Cao WD, Zhang X, Zhang JN et al (2006) Immunocytochemical detection of 14-3-3 in primary nervous system tumors. J Neurooncol 77:125–130
Yang X, Cao W, Lin H et al (2009) Isoform-specific expression of 14-3-3 proteins in human astrocytoma. J Neurol Sci 276:54–59
Sunayama J, Tsuruta F, Masuyama N et al (2005) JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol 170:295–304
Yang X, Cao W, Zhang L et al (2012) Targeting 14-3-3zeta in cancer therapy. Cancer Gene Ther 19:153–159
Neal CL, Xu J, Li P et al (2012) Overexpression of 14-3-3ζ in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene 31:897–906
Karar J, Gerniglia GJ, Lindsten T et al (2012) Dual PI3K/mTOR inhibitor NVP-BEZ235 suppresses hypoxia-inducible factor (HIF)-1α expression by blocking protein translocation and increases cell death under hypoxia. Cancer Biol Ther 13:1102–1111
Lin YM, Huang YL, Fong YC et al (2012) Hepatocyte growth factor increases vascular endothelial growth factor-A production in human synovial fibroblasts through c-Met receptor pathway. PLoS One 7:e50924. doi:10.1371/journal.pone.0050924
Remmele W, Hildebrand U, Hienz HA et al (1986) Comparative histological, histochemical, immunohistochemical and biochemical studies on estrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch 409:127–147
Cao L, Cao W, Zhang W et al (2008) Identification of 14-3-3 protein isoforms in human astrocytoma by immunohistochemistry. Neurosci Lett 432:94–99
Aitken A (2006) 14-3-3 proteins: a historic overview. Semin Cancer Biol 16:162–172
Berardi S, Caivano A, Ria R et al (2012) Four proteins governing overangiogenic endothelial cell phenotype in patients with multiple myeloma are plausible therapeutic targets. Oncogene 31:2258–2269
Kobayashi H, Ogura Y, Sawada M et al (2011) Involvement of 14-3-3 proteins in the second epidermal growth factor-induced wave of Rac1 activation in the process of cell migration. J Biol Chem 286:39259–39268
Oksvold MP, Huitfeldt HS, Langdon WY (2004) Identification of 14-3-3zeta as an EGF receptor interacting protein. FEBS Lett 569:207–210
Cao W, Yang X, Zhou J et al (2010) Targeting 14-3-3 protein, difopein induces apoptosis of human glioma cells and suppresses tumor growth in mice. Apoptosis 15:230–241
Yang X, Cao W, Zhou J et al (2011) 14-3-3zeta positive expression is associated with a poor prognosis in patients with glioblastoma. Neurosurgery 68:932–938
Jensen RL (2009) Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol 92:317–335
Kaur B, Khwaja FW, Severson EA et al (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol 7:134–153
Oliver L, Olivier C, Marhuenda FB et al (2009) Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol 2:263–284
Mashiko R, Takano S, Ishikawa E et al (2011) Hypoxia-inducible factor 1α expression is a prognostic biomarker in patients with astrocytic tumors associated with necrosis on MR image. J Neurooncol 102:43–50
Gillespie DL, Whang K, Ragel BT et al (2007) Silencing of hypoxia inducible factor-1alpha by RNA interference attenuates human glioma cell growth in vivo. Clin Cancer Res 13:2441–2448
Argyriou AA, Giannopoulou E, Kalofonos HP (2009) Angiogenesis and anti-angiogenic molecularly targeted therapies in malignant gliomas. Oncology 77:1–11
Machein MR, Plate KH (2000) VEGF in brain tumors. J Neurooncol 50:109–120
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research (C) (21591845) and (B) (22390277) from the Ministry of Education, Science and Culture of Japan. Wei-Dong Cao is a research doctor from the Department of Neurosurgery, Xijing Hospital, Fourth Military Medical University, China. This study was also supported by a Grant in China (National Nature Science Foundation of China, no: 81072083).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cao, WD., Kawai, N., Miyake, K. et al. Relationship of 14-3-3zeta (ζ), HIF-1α, and VEGF expression in human brain gliomas. Brain Tumor Pathol 31, 1–10 (2014). https://doi.org/10.1007/s10014-013-0135-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-013-0135-3