Abstract
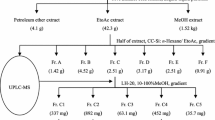
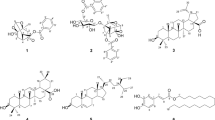
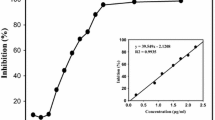
In the current study, our investigation of inhibitory compounds from the ethyl acetate-soluble fraction of 95% ethanol extract of Thunberg’s Geranium (Geranium thunbergii Sieb. et Zucc.; TG) was followed by identification of the inhibitory compounds by a combination of HPLC microfractionation and an enzyme assay in a 96-well plate. Structural analyses of the active compounds were carried out using LC-MSn. The main compound inhibiting yeast α-glucosidase in this plant was tentatively identified as geraniin by LC-MSn. Six compounds, p-hydroxybenzoic acid (1) (IC50 >362.32 μM), brevifolin carboxylic acid (2) (IC50 > 171.23 μM), geraniin (3) (IC50 =4.09 μM), ellagic acid (4) (IC50 = 63.91 μM), kaempferol-3-O-arabinofuranosyl-7-O-rhamnopyranoside (5) (IC50 =27.70 μM), and kaempferitrin (6) (IC50 > 86.51 μM) were isolated from the active EtOAc-soluble fraction of TG on yeast α-glucosidase. However, the 6 isolated compounds were very weak inhibitory activity against mammalian α-glucosidase and α-amylase. This study suggests that the developed HPLC microfractionation with an enzyme assay system is one of the powerful tools for rapid screening and identification of enzyme inhibitors in complex extracts.
Similar content being viewed by others
References
Queiroz EF, Wolfender JL, Atindehou KK, Taore D, Hostettmann K. On-line identification of the antifungal constituents of Erythrina vogelii by liquid chromatography with tandem mass spectrometry, ultraviolet absorbance detection, and nuclear magnetic resonance spectrometry combined with liquid chromatographic microfractionation. J. Chromatogr. A 974: 123–134 (2002)
Fitch RW, Garraffo HM, Spande TF, Yeh HJ, Daly JW. Bioassayguided isolation of epiquinamide, a novel quinolizidine alkaloid, and nicotinic agonist from an Ecuadoran poison frog, Epipedobates tricolor. J. Nat. Prod. 66: 1345–1350 (2003)
Wennberg T, Kreander K, Lahdevuori M, Vuorela H, Vuorela P. Strategy for primary screening of natural products using micro fractionation combined with a bioassay. J. Liq. Chromatogr. R. T. 27: 2571–2590 (2004)
Braun C, Brayer GD, Withers SG. Mechanism-based inhibition of yeast α-glucosidase and human pancreatic α-amylase by a new class of inhibitors. 2-Deoxy-2,2-difluoro-a-glycosides. J. Biol. Chem. 270: 26778–26781 (1995)
Dwek RA, Butters TD, Platt FM, Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 1: 65–75 (2002)
Matsumoto K, Yano M, Miyake S, Ueki Y, Yamaguchi Y, Akazawa S, Tominaga Y. Effects of voglibose on glycemic excursions, insulin secretion, and insulin sensitivity in non-insulin-treated NIDDM patients. Diabetes Care 21: 256–260 (1998)
Murai A, Iwamura K, Takada M, Ogawa K, Usui T, Okumura J. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sci. 71: 1405–1415 (2002)
Hollander P. Safety profile of acarbose, an α-glucosidase inhibitor. Drugs 44: 47–53 (1992)
Gao H, Huang YN, Xu PY, Kawabata J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 105: 628–634 (2007)
Mcdougall GJ, Shpiro F, Doboson P, Smith P, Blacke A, Stewart D. Different polyphenolic compounds of soft fruits inhibit α-amylase and α-glucosidase. J. Agr. Food Chem. 53: 2760–2766 (2005)
Ito H, Hatano T, Namba O, Shirono T, Okuda T, Yoshida T. Constituents of Geranium thunbergii Sieb. Et Zucc. XV. Modified dehydroellagitannins, geraniinic acids B and C, and phyllanthusiin F. Chem. Pharm. Bull. 47: 1148–1151 (1999)
Okuda T, Yoshida T, Hatano T, Ikeda Y, Shingu T, Inoue T. Constituents of Geranium thunbergii Sieb. et Zucc. XlII. Isolation of water-soluble tannins by centrifugal partition chromatography, a biomimetic synthesis of elaeocarpusin. Chem. Pharm. Bull. 34: 4075–4082 (1986)
Matsui T, Yoshimoto C, Osajima K, Oki T, Osajima Y. In vitro survey of α-glucosidase inhibitory food components. Biosci. Biotech. Bioch. 60: 2019–2022 (1996)
Adachi TMC, Sakurai K, Shihara N, Tsuda K, Yasuda K. Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. J. Endocrinol. 50: 271–279 (2003)
Gao H, Kawabata J. α-Glucosidase inhibition of 6-hydroxyflavones. Part 3: Synthesis and evaluation of 2,3,4-trihydroxybenzoyl containing flavonoid analogs and 6-aminoflavones as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 3: 1661–1671 (2005)
Zhang JH, Chung TD, Oldenburg KR. A simple statistical prarameter for use in evaluation and validation of high troughput screening assay. J. Biomol. Screen. 4: 67–73 (1999)
Bollini S, Herbst JJ, Gaughan GT, Verdoorn TA, Ditta J, Dubowchik GM, Vinitsky A. High-throughput fluorescence polarization method for identification of FKBP12 ligands. J. Biomol. Screen. 7: 526–530 (2002)
Lineweaver H, Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56: 658–666 (1934)
Zhang LZ, Guo YJ, Tu GZ, Guo WB, Miao F. Isolation and identification of a novel ellagitannin from Phyllnthus uriaria L. Acta Pharm. Sin. 39: 119–112 (2004)
Kwon OG, Kim SH, Chun BY, Park DK, Son KH. Isolation of antimicrobial components from Montan Cortex. Korean J. Physiol. Pha. 30: 340–344 (1999)
Kitagawa K, Kawamoto T, Futaki S, Kiyama S, Akita T, Moritoki H, Kiso Y. Solution syntheses of two enkephalin-containing peptides E and dynorphin (1–24), using Nin-(2,4,6-triisopropylphenylsulfonyl) trytophan. Chem. Pharm. Bull. 37: 2631–2638 (1989)
Yisida T, Itoh H, Matsunaga S, Tanaka R, Okuda T. Tannins and related polyphenols of euphobiaceous plants. IX. Hydorlyzable tannins with 1C-glucose core from Phyllanthus flexuosus Muell. Chem. Pharm. Bull. 40: 520–521 (1992)
Nawwar MAM, Hussein SSM, Merfort I. NMR spectral analysis of polyphenols from Punica Granatum. Pytochemistry 36: 793–768 (1994)
Yonekura-Sakakibara K, Tohge T, Matsuda F, Nakabayashi R, Takayama H, Niida R, Watanabe-Takahashi A, Inoue E, Saito K. Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene-metabolite correlations in Arabidopsis. Plant Cell 20: 2160–2176 (2008)
Mulinacci N, Vincicieri FF, Baldi A, Bambagiotti AM, Sendi A, Wagner H. Flavonol glycosides from Sedum telephium subspecies maximum leaves. Phytochemistry 38: 531–533 (1995)
Chethan S, Shylaja MD, Nagappa GM. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg. Med. Chem. 16: 10085–10090 (2008)
Gunawan-Puteri MDPT, Kawabata J. Novel α-glucosidase inhibitors from Macaranga tanarius leaves. Food Chem. 123: 384–389 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.J., Kim, J.K., Jang, J.M. et al. Rapid identification of the α-glucosidase inhibitory compounds from Thunberg’s Geranium (Geranium thunbergii Sieb. et Zucc.). Food Sci Biotechnol 21, 987–996 (2012). https://doi.org/10.1007/s10068-012-0129-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-012-0129-7