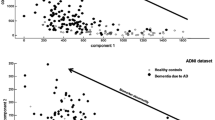
Abstract
The neuropathological processes eventually leading to Alzheimer’s disease (AD) are thought to start decades before the appearance of clinical symptoms and the clinical diagnosis of AD dementia. The term “preclinical AD” has been recently introduced to identify this “silent stage” of AD, when the disease is already present, but symptoms are not yet clinically evident. Advances in AD biomarkers have dramatically improved the ability to detect AD pathological processes in vivo in cognitively intact subjects, thus demonstrating the presence of AD pathology in the preclinical phase. This review focuses on the recent advances in the field of neuroimaging and CSF AD biomarkers specifically in the preclinical phase of AD, and aims to discuss the significance that such biomarkers could have in cognitively intact subjects. Even though the use of such biomarkers in AD preclinical phase has contributed to improve our understanding of AD early pathological processes, it raised also a number of new challenges that still remain to be overcome, such as a better definition of the clinical and individual significance of currently known biomarkers in preclinical stages and the development of novel biomarkers of different early AD-related events.





Similar content being viewed by others
References
Sperling RA, Aisen PS, Beckett LA et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7:280–292. doi:10.1016/j.jalz.2011.03.003
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. NBA 18:351–357
Jack CR Jr, Knopman DS, Weigand SD et al (2012) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71:765–775. doi:10.1002/ana.22628
Salmon DP, Ferris SH, Thomas RG et al (2013) Age and apolipoprotein E genotype influence rate of cognitive decline in nondemented elderly. Neuropsychology 27:391–401. doi:10.1037/a0032707
Blennow K (2010) Biomarkers in Alzheimer’s disease drug development. Nat Med 16:1218–1222. doi:10.1038/nm.2221
Knopman DS, Jack CR, Wiste HJ et al (2012) Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 78:1576–1582. doi:10.1212/WNL.0b013e3182563bbe
Group BDW (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. doi:10.1067/mcp.2001.113989
Pupi A, Mosconi L, Nobili FM, Sorbi S (2005) Toward the validation of functional neuroimaging as a potential biomarker for Alzheimer’s disease: implications for drug development. Mol Imaging Biol 7:59–68. doi:10.1007/s11307-005-0953-8
Hampel H, Lista S (2013) Use of biomarkers and imaging to assess pathophysiology, mechanisms of action and target engagement. J Nutr Health Aging 17:54–63. doi:10.1007/s12603-013-0003-1
Vellas B, Carrillo MC, Sampaio C et al (2013) Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. In: Alzheimers Dement. pp 438–444
Parekh A, Buckman-Garner S, McCune S et al (2015) Catalyzing the Critical Path Initiative: fDA’s progress in drug development activities. Clin Pharmacol Ther 97:221–233. doi:10.1002/cpt.42
Jack CR, Knopman DS, Jagust WJ et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. doi:10.1016/S1474-4422(12)70291-0
Mattsson N, Zetterberg H, Hansson O et al (2009) CSF biomarkers and incipient alzheimer disease in patients with mild cognitive impairment. JAMA 302:385–393. doi:10.1001/jama.2009.1064
Klunk WE, Engler H, Nordberg A, Wang Y (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B—Klunk—2004—Annals of Neurology—Wiley Online Library. Annals of …
Jagust WJ, Bandy D, Chen K et al (2010) The Alzheimer’s disease neuroimaging initiative positron emission tomography core. Alzheimers Dement 6:221–229. doi:10.1016/j.jalz.2010.03.003
Dickerson BC, Wolk DA, Alzheimer’s Disease Neuroimaging Initiative (2012) MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 78:84–90. doi:10.1212/WNL.0b013e31823efc6c
Alzheimer’s Association (2015) Alzheimer’s disease facts and figures. Alzheimers Dement 11:332–384
Bertram L, Lill CM, Tanzi RE (2010) The genetics of Alzheimer disease: back to the future. Neuron 68:270–281. doi:10.1016/j.neuron.2010.10.013
Gee JR, Keller JN (2005) Astrocytes: regulation of brain homeostasis via apolipoprotein E. Int J Biochem Cell Biol 37:1145–1150. doi:10.1016/j.biocel.2004.10.004
Farrer LA, Cupples LA, Haines JL et al (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis Consortium. JAMA 278:1349–1356
Mosconi L, Berti V, Swerdlow RH et al (2010) Maternal transmission of Alzheimer’s disease: prodromal metabolic phenotype and the search for genes. Hum Genomics 4:170–193
Gómez-Tortosa E, Barquero MS, Barón M et al (2007) Variability of age at onset in siblings with familial Alzheimer disease. Arch Neurol 64:1743–1748. doi:10.1001/archneur.64.12.1743
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368
Sperling RA, Johnson KA (2013) Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum 19:325–338
Stern Y (2009) Cognitive reserve. Neuropsychologia 47:2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004
Ewers M, Insel PS, Stern Y et al (2013) Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology 80:1194–1201. doi:10.1212/WNL.0b013e31828970c2
Castellani RJ, Perry G (2014) The complexities of the pathology–pathogenesis relationship in Alzheimer disease. Biochem Pharmacol 88:671–676. doi:10.1016/j.bcp.2014.01.009
Vlassenko AG, Mintun MA, Xiong C et al (2011) Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C] Pittsburgh compound B data. Ann Neurol 70:857–861. doi:10.1002/ana.22608
Kundaikar HS, Degani MS (2015) Insights into the interaction mechanism of ligands with Aβ42 based on molecular dynamics simulations and mechanics: implications of role of common binding site in drug design for Alzheimer’s disease. Chem Biol Drug Des. doi:10.1111/cbdd.12555
Vallabhajosula S (2011) Positron emission tomography radiopharmaceuticals for imaging brain beta-amyloid. Semin Nucl Med 41:283–299. doi:10.1053/j.semnuclmed.2011.02.005
Shoghi-Jadid K, Small GW, Agdeppa ED et al (2002) Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with alzheimer disease. Am J Geriatr Psychiatry 10:24–35. doi:10.1097/00019442-200201000-00004
Rowe CC, Ackerman U, Browne W et al (2008) Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 7:129–135. doi:10.1016/S1474-4422(08)70001-2
Koole M, Lewis DM, Buckley C et al (2009) Whole-body biodistribution and radiation dosimetry of 18F-GE067: a radioligand for in vivo brain amyloid imaging. J Nucl Med 50:818–822. doi:10.2967/jnumed.108.060756
Kung HF, Choi SR, Qu W et al (2010) 18F stilbenes and styrylpyridines for PET imaging of A beta plaques in Alzheimer’s disease: a miniperspective. J Med Chem 53:933–941. doi:10.1021/jm901039z
Sabri O, Seibyl J, Rowe C, Barthel H (2015) Beta-amyloid imaging with florbetaben. Clin Transl Imaging 3:13–26. doi:10.1007/s40336-015-0102-6
Mintun MA, Larossa GN, Sheline YI et al (2006) [11C] PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67:446–452. doi:10.1212/01.wnl.0000228230.26044.a4
Aizenstein HJ, Nebes RD, Saxton JA et al (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65:1509–1517. doi:10.1001/archneur.65.11.1509
Rowe CC, Ellis KA, Rimajova M et al (2010) Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging 31:1275–1283. doi:10.1016/j.neurobiolaging.2010.04.007
Jagust WJ, Mormino EC (2011) Lifespan brain activity. Trends Cognit Sci 15:520–526. doi:10.1016/j.tics.2011.09.004
Schott JM, Bartlett JW, Fox NC et al (2010) Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Ann Neurol 68:825–834. doi:10.1002/ana.22315
Resnick SM, Sojkova J, Zhou Y et al (2010) Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 74:807–815. doi:10.1212/WNL.0b013e3181d3e3e9
Chételat G, Villemagne VL, Pike KE et al (2011) Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease. Brain 134:798–807. doi:10.1093/brain/awq383
Villain N, Chételat G, Grassiot B et al (2012) Regional dynamics of amyloid-β deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: a voxelwise PiB-PET longitudinal study. Brain 135:2126–2139. doi:10.1093/brain/aws125
Knight WD, Okello AA, Ryan NS et al (2011) Carbon-11-Pittsburgh compound B positron emission tomography imaging of amyloid deposition in presenilin 1 mutation carriers. Brain 134:293–300. doi:10.1093/brain/awq310
Villemagne VL, Ataka S, Mizuno T et al (2009) High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol 66:1537–1544. doi:10.1001/archneurol.2009.285
Berti V, Nacmias B, Bagnoli S, Sorbi S (2011) Alzheimer’s disease: genetic basis and amyloid imaging as endophenotype. Q J Nucl Med Mol Imaging 55:225–236
Reiman EM, Chen K, Liu X et al (2009) Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci 106:6820–6825. doi:10.1073/pnas.0900345106
Fleisher AS, Chen K, Liu X et al (2013) Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 34:1–12. doi:10.1016/j.neurobiolaging.2012.04.017
Mosconi L, Rinne JO, Tsui WH et al (2010) Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci 107:5949–5954. doi:10.1073/pnas.0914141107
Lee H-G, Casadesus G, Zhu X et al (2004) Challenging the amyloid cascade hypothesis: senile plaques and amyloid-beta as protective adaptations to Alzheimer disease. Ann NY Acad Sci 1019:1–4. doi:10.1196/annals.1297.001
Pimplikar SW, Nixon RA, Robakis NK et al (2010) Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J Neurosci 30:14946–14954. doi:10.1523/JNEUROSCI.4305-10.2010
Herrup K (2010) Reimagining Alzheimer’s disease—an age-based hypothesis. J Neurosci 30:16755–16762. doi:10.1523/JNEUROSCI.4521-10.2010
Hyman BT, Phelps CH, Beach TG et al (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. doi:10.1016/j.jalz.2011.10.007
Klunk WE, Koeppe RA, Price JC et al (2015) The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 11(1–15):e1–e4. doi:10.1016/j.jalz.2014.07.003
Mosconi L (2005) Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging 32:486–510. doi:10.1007/s00259-005-1762-7
Mosconi L, Mistur R, Switalski R et al (2009) FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging 36:811–822. doi:10.1007/s00259-008-1039-z
Mosconi L, De santi S, Li J et al (2008) Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging 29:676–692. doi:10.1016/j.neurobiolaging.2006.12.008
Nacmias B, Berti V, Piaceri I, Sorbi S (2013) FDG PET and the genetics of dementia. Clin Transl Imaging 1:235–246. doi:10.1007/s40336-013-0028-9
Mosconi L, Sorbi S, De Leon MJ et al (2006) Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 47:1778–1786
Jagust WJ, Landau SM, For the Alzheimer’s Disease Neuroimaging Initiative (2012) Apolipoprotein E, not fibrillar—amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci 32:18227–18233. doi:10.1523/JNEUROSCI.3266-12.2012
Mosconi L, Brys M, Switalski R et al (2007) Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci 104:19067–19072. doi:10.1073/pnas.0705036104
Stern Y, Alexander GE, Prohovnik I, Mayeux R (1992) Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 32:371–375. doi:10.1002/ana.410320311
Berti V, Vanzi E, Polito C, Pupi A (2013) Back to the future: the absolute quantification of cerebral metabolic rate of glucose. Clin Transl Imaging 1:289–296. doi:10.1007/s40336-013-0030-2
Herholz K (2014) The role of PET quantification in neurological imaging: fDG and amyloid imaging in dementia. Clin Transl Imaging 2:321–330. doi:10.1007/s40336-014-0073-z
Dubois B, Feldman HH, Jacova C, et al. (2010) Revising the definition of Alzheimer’s disease: a new lexicon. In: Lancet Neurol. pp 1118–1127
Shaw LM, Vanderstichele H, Knapik-Czajka M et al (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65:403–413. doi:10.1002/ana.21610
Blennow K, Dubois B, Fagan AM et al (2015) Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement 11:58–69. doi:10.1016/j.jalz.2014.02.004
Buchhave P, Minthon L, Zetterberg H et al (2012) Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 69:98–106. doi:10.1001/archgenpsychiatry.2011.155
Braak H, Zetterberg H, Del Tredici K, Blennow K (2013) Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol 126:631–641. doi:10.1007/s00401-013-1139-0
Tolboom N, van der Flier WM, Yaqub M et al (2009) Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 50:1464–1470. doi:10.2967/jnumed.109.064360
Toledo JB, Bjerke M, Da X et al (2015) Nonlinear association between cerebrospinal fluid and florbetapir F-18 β-amyloid measures across the spectrum of alzheimer disease. JAMA Neurol 72:571–581. doi:10.1001/jamaneurol.2014.4829
Buerger K, Ewers M, Pirttilä T et al (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129:3035–3041. doi:10.1093/brain/awl269
Sämgård K, Zetterberg H, Blennow K et al (2010) Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int J Geriatr Psychiatry 25:403–410. doi:10.1002/gps.2353
Tarawneh R, Head D, Allison S et al (2015) Cerebrospinal fluid markers of neurodegeneration and rates of brain atrophy in early alzheimer disease. JAMA Neurol 72:656–665. doi:10.1001/jamaneurol.2015.0202
Jansen WJ, Ossenkoppele R, Knol DL et al (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313:1924–1938. doi:10.1001/jama.2015.4668
Ritchie C, Smailagic N, Noel-Storr AH et al (2014) Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 6:CD008782. doi:10.1002/14651858.CD008782.pub4
Mattsson N, Insel PS, Donohue M et al (2015) Predicting reduction of cerebrospinal fluid β-amyloid 42 in cognitively healthy controls. JAMA Neurol 72:554–560. doi:10.1001/jamaneurol.2014.4530
Schoonenboom NSM, Reesink FE, Verwey NA et al (2012) Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78:47–54. doi:10.1212/WNL.0b013e31823ed0f0
Vos SJ, Xiong C, Visser PJ et al (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol 12:957–965. doi:10.1016/S1474-4422(13)70194-7
Slaets S, Le Bastard N, Theuns J et al (2013) Amyloid pathology influences aβ1-42 cerebrospinal fluid levels in dementia with lewy bodies. J Alzheimers Dis 35:137–146. doi:10.3233/JAD-122176
Koopman K, Le Bastard N, Martin J-J et al (2009) Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau(181P). Neurochem Int 55:214–218. doi:10.1016/j.neuint.2009.02.017
Mattsson N, Andreasson U, Persson S et al (2013) CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement 9:251–261. doi:10.1016/j.jalz.2013.01.010
Ferreira D, Rivero-Santana A, Perestelo-Pérez L et al (2014) Improving CSF biomarker’ performance for predicting progression from mild cognitive impairment to Alzheimer’s disease by considering different confounding factors: a meta-analysis. Front Aging Neurosci 6:287. doi:10.3389/fnagi.2014.00287
Yang Y, Cui M (2014) Radiolabeled bioactive benzoheterocycles for imaging β-amyloid plaques in Alzheimer’s disease. Eur J Med Chem 87:703–721. doi:10.1016/j.ejmech.2014.10.012
Okamura N, Harada R, Furumoto S et al (2014) Tau PET imaging in Alzheimer’s disease. Curr Neurol Neurosci Rep 14:500. doi:10.1007/s11910-014-0500-6
Harada R, Okamura N, Furumoto S, et al. (2015) 18F-THK5351: A Novel PET Radiotracer for Imaging Neurofibrillary Pathology in Alzheimer’s Disease. J Nucl Med Nov 5. pii: jnumed.115.164848. [Epub ahead of print]
Castellani RJ, Perry G (2014) The complexities of the pathology–pathogenesis relationship in Alzheimer disease. Biochem Pharmacol 88:671–676. doi:10.1016/j.bcp.2014.01.009
Janssen B, Vugts DJ, Funke U et al (2015) Imaging of neuroinflammation in Alzheimer’s disease, multiple sclerosis and stroke: recent developments in positron emission tomography. Biochim Biophys Acta. doi:10.1016/j.bbadis.2015.11.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Berti, V., Polito, C., Lombardi, G. et al. Rethinking on the concept of biomarkers in preclinical Alzheimer’s disease. Neurol Sci 37, 663–672 (2016). https://doi.org/10.1007/s10072-016-2477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2477-1