Abstract
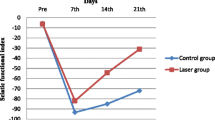
Peripheral nerves are structures that, when damaged, can result in significant motor and sensory disabilities. Several studies have used therapeutic resources with the aim of promoting early nerve regeneration, such as the use of low-power laser. However, this laser therapy does not represent a consensus regarding the methodology, thus yielding controversial conclusions. The objective of our study was to investigate, by functional evaluation, the comparative effects of low-power laser (660 nm and 830 nm) on sciatic nerve regeneration following crushing injuries. Twenty-seven Wistar rats subjected to sciatic nerve injury were divided into three groups: group sham, consisting of rats undergoing simulated irradiation; a group consisting of rats subjected to gallium–aluminum–arsenide (GaAlAs) laser at 660 nm (10 J/cm2, 30 mW and 0.06 cm2 beam), and another one consisting of rats subjected to GaAlAs laser at 830 nm (10 J/cm2, 30 mW and 0.116 cm2). Laser was applied to the lesion for 21 days. A sciatic functional index (SFI) was used for functional evaluation prior to surgery and on days 7, 14, and 21 after surgery. Differences in SFI were found between group 660 nm and the other ones at the 14th day. One can observe that laser application at 660 nm with the parameters and methods utilised was effective in promoting early functional recovery, as indicated by the SFI, over the period evaluated.


Similar content being viewed by others
References
Ferrigno ISV, Freitas PP, Freitas AD (2005) Peripheral nerve injuries. In: Freitas PP (ed) Hand rehabilitation, 1st edn. Atheneu, São Paulo, pp 211–254
Noble J, Munro CA, Prasad VS, Midha R (1998) Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45:116–122
Rodrígues FJ, Valero-Cabré A, Navarro X (2004) Regeneration and functional recovery following peripheral nerve injury. Drug Discov Today Dis Models 1:177–185
Lundborg G (2000) A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg [Am] 25:391–414
Fonseca MCR, Mazzer N, Barbieri CH, Elui VMC (2006) Hand trauma: retrospective study. Rev Bras Ortop 41:181–186
Novak CB, Mackinnon SE (2005) Evaluation of nerve injury and nerve compression in the upper quadrant. J Hand Ther 18:230–240
Marcolino AM, Barbosa RI, Fonseca MCR, Mazzer N, Elui VMC (2008) Physical therapy in brachial plexus injury: case report. Rev Fisioter Mov 21:53–61
Sulaiman OA, Gordon T (2000) Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia 32:234–46
Dahlin LB (2004) The biology of nerve injury and repair. J Am Soc Surg Hand 4:143–155
Bell JA, Groswald DE, Luttges MW (1984) Alterations in the mechanical properties of peripheral nerve following crush injury. J Biomech 17:185–193
Sunderland S (1990) The anatomy and physiology of nerve injury. Muscle Nerve 13:771–784
Mendonça AC, Barbieri CH, Mazzer N (2003) Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods 129:183–190
Monte-raso VV, Barbieri CH, Mazzer N, Fazan VS (2005) Can therapeutic ultrasound influence the regeneration of peripheral nerves? J Neurosci Methods 142:185–192
Endo C, Barbieri CH, Mazzer N, Fazan VS (2008) Low-power laser therapy accelerates peripheral nerves' regeneration. Acta Ortop Bras 16:305–310
Belchior ACG, Reis FA, Nicolau RA, Silva IS, Pereira DM, Carvalho PTC (2009) Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 24:893–899
Reis FA, Belchior ACG, Carvalho PTC, Silva BAK, Pereira DM, Silva IS, Nicolau RA (2009) Effects of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24:741–747
Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE (2007) Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg 25:436–442
Rochkind S, Leider-trejo L, Nissan M, Shamir MH, Kharenko O, Alon M (2007) Efficacy of 780 nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed Laser Surg 25:137–143
Rochkind S, Nissan M, Alon M, Shamir M, Salame K (2001) Effects of laser irradiation on the spinal cord for the regeneration of crushed peripheral nerve in rats. Lasers Surg Med 28:216–219
Kitchen SS, Partridge CJ (1991) A review of low level laser therapy, part I: background, physiological effects and hazards. Physiotherapy 77:161–163
Karu TI, Pyatibrat L, Kalendo G (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27:219–33
Khullar SM, Brodin P, Fristad I, Kvinnsland IH (1999) Enhanced sensory reinnervation of dental target tissues in rats following low level laser (LLL) irradiation. Lasers Med Sci 14:177–184
Schindl A, Schindl M, Schindl L, Jurecka W, Hönigsmann H, Breier F (1999) Increased dermal angiogenesis after low-intensity laser therapy for a chronic radiation ulcer determined by a video measuring system. J Am Acad Dermatol 40:481–484
Manteifel V, Bakeeva L, Karu T (1997) Ultrastructural changes in chondriome of human lymphocytes after irradiation with He-Ne laser: appearance of giant mitochondria. J Photochem Photobiol B 38:25–30
Enwemeka CS (2009) Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg 27:387–393
Chen YS, Hsu SF, Chiu CW, Lin JG, Chen CT, Yao CT (2005) Effects of low-power pulsed laser on peripheral nerve regeneration in rats. Microsurgery 25:83–89
Gigo-Benato D, Geuna S, Rodrigues AC, Fornaro PTM, Boux E, Battiston B, Giacobini-Robecchi MG (2004) Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: a double-blind randomized study in the rat median nerve model. Lasers Med Sci 19:57–65
Stainki DR, Raiser AG, Graça DL, Becker C, Fernandez GMS (1999) Gallium arsenide (GaAs) laser radiation in the radial nerve regeneration submitted secondary to surgical repair. Braz J Vet Res Anim Sci 35:37–40
Ozen T, Orhan K, Gorur I, Ozturk A (2006) Efficacy of low level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head Face Medicine 2:3
Khullar SM, Brodin P, Messelt EB, Haanaes HR (1995) The effects of low level laser treatment on recovery of nerve conduction and motor function after compression injury in the rat sciatic nerve. Eur J Oral Sci 103:299–305
de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77:634–643
Gasparini ALP, Barbieri CH, Mazzer N (2007) Correlation between different methods of gait functional evaluation in rats with ischiatic nerve crushing injuries. Acta Ortop Bras 15:285–289
Bain JR, Mackinnon SE, Hunter RT (1989) Functional evaluation of complete sciatic, peroneal and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129–138
Pachioni CAS, Mazzer N, Barbieri CH, Fazan VPS, Moro CA, Silva CAA (2006) Rats’ ischiatic nerve injury caused by smashing: a vascularization study. Acta Ortop Bras 14:203–207
Mazzer PYCN, Barbieri CH, Mazzer N, Fazan VPS (2008) Morphologic and morphometric evaluation of experimental acute crush injuries of the sciatic nerve of rats. J Neurosci Methods 173:249–258
Mclean RA, Sanders WL, Stroup WW (1991) A unified approach to mixed linear models. Am Stat 5:54–64
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute Inc, Cary
SAS/STAT® (2002–2003) User’s guide, version 9. SAS Institute Inc, Cary
Mazzer PYCN, Barbieri CH, Mazzer N, Fazan VPS (2006) Qualitative and quantitative evaluation of rats acute injuries caused by ischiatic nerve smashing. Acta Ortop Bras 14:220–225
Rochkind S, Barrnea L, Razon N, Bartal A, Schwartz M (1987) Stimulatory effect of He-Ne low dose laser on injured sciatic nerves of rats. Neurosurgery 20:843–847
Walsh DM, Baxter GD, Allen JM (2000) Lack of effect of pulsed low-intensity infrared (820 nm) laser irradiation on nerve conduction in the human superficial radial nerve. Lasers Surg Med 26:485–490
Mohammed IFR, AL-Mustawfi NBV, Kaka LN (2007) Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomed Laser Surg 25:107–111
Gigo-Benato D, Geuna S, Rochkind S (2005) Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve 31:694–701
Bagis S, Comelekoglu U, Sahin G, Buyukakilli B, Erdogan C, Kanik A (2002) Acute electrophysiologic effect of pulsed gallium–arsenide low energy laser irradiation on configuration of compound nerve action potential and nerve excitability. Lasers Surg Med 30:376–380
Amat A, Rigau J, Waynant RW, Ilev IK, Anders JJ (2006) The electric field induced by light can explain cellular responses to electromagnetic energy: a hypothesis of mechanism. J Photochem Photobiol B 82:152–60
Vladimirov Yu A (1994) In: Chikin S (ed) Efferent medicine. Institute of Biomedical Chemistry, Russian Academy of Medical Sciences, Moscow, pp. 51–66
Vladimirov Yu A, Osipov AN, Klebanov GI (2004) Photobiological principles of therapeutic applications of laser radiation. Biochemistry 69:81–90
Romm AR, Sherstnev MP, Volkov VV, Vladimirov Yu A (1986) The action of laser irradiation of peroxide chemiluminescence of wound exudation. Byul Eksp Biol Med 102:426–428
Pedersen PL, Carafoli E (1987) Ion motive ATPase. I. Ubiquity, properties and significance to cell function. Trends Biochem Sci 12:146–150, 186–189
Manteifel VM, Karu TI (2005) Structure of mitochondria and activity of their respiratory chain in successive generations of yeast cells exposed to He-Ne laser light. Izv Akad Nauk Ser Biol 32:556–566
Kilanczyk E, Palecz D, Bryszewska M (2002) Effect of red laser light on Na+–K+-ATPase activity in human erythrocyte membranes sensitized with Zn-phthalocyanine. J Clin Laser Med Surg 20:71–75
Karu TI, Pyatibrat LV, Afanasyeva NI (2004) A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol 80:366–372
Bolognani L, Cavalca M, Magnani C, Volpi N (1992) ATP synthesis catalysed by myosin ATPase: effect of laser and e.m. field. Laser Technol 2:115–120
Schwartz F, Brodie C, Appel E, Kazimirsky G, Shainberg A (2002) Effect of helium/neon laser irradiation on nerve growth factor synthesis and secretion in skeletal muscle cultures. J Photochem Photobiol B 66:195–200
Gulsoy M, Ozer GH, Bozkulak O, Tabakoglu HO, Aktas E, Deniz G, Ertan C (2006) The biological effects of 632.8-nm low energy He-Ne laser on peripheral blood mononuclear cells in vitro. J Photochem Photobiol B 82:199–202
Karu T (1992) Derepression of the genome after irradiation of human lymphocytes with HeNe laser. Laser Therapy 4:5–24
Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G (2008) Is the sciatic functional index always reliable and reproducible? J Neurosci Methods 170:255–261
Acknowledgements
This project had financial support from the State of São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP), Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbosa, R.I., Marcolino, A.M., de Jesus Guirro, R.R. et al. Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25, 423–430 (2010). https://doi.org/10.1007/s10103-009-0750-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0750-8