Abstract
Background
The neutrophil–lymphocyte ratio (NLR) is associated with a poor prognosis in many cancers but the biological mechanisms involved are unknown. Since cytokines and angiogenic factors (CAFs) are reflected by various immune responses, we analyzed the association between the NLR and CAFs and their prognostic implications in gastric cancer (GC).
Methods
Of 745 GC patients who were enrolled in NLR analysis, 70 underwent NLR and CAF association analyses. Pretreatment serum levels of 52 CAFs were measured by means of multiplex bead immunoassays and enzyme-linked immunosorbent assays. Linear regression analysis and survival analysis of the NLR with each CAF were performed.
Results
Metastatic organ numbers and carbohydrate antigen 19-9 levels were significantly higher in patients with a high NLR [greater than 2.42 (median): P = 0.047 and P < 0.001 respectively]. The overall survival was significantly worse in the high NLR group (17.8 months vs 11.2 months, P < 0.001). In CAF analysis, osteopontin (R 2 = 0.337, P < 0.001) and interleukin-6 (R 2 = 0.141, P = 0.001) were significantly associated with the NLR. Stromal-cell-derived factor 1 (SDF-1) was a significant poor prognostic factor independently of the NLR. Consideration of both the NLR and SDF-1 divided patient groups with different overall survival (both low, 21.0 months; either high, 15.8 months; both high, 8.2 months).
Conclusion
The NLR is a significant poor prognostic factor in advanced GC. The NLR is mainly associated with osteopontin and interleukin-6. Besides the NLR, SDF-1 is an independent poor prognostic factor in GC. Consideration of both the NLR and SDF-1 might give insights into antitumor immunity in GC.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the third leading cause of cancer deaths worldwide [1]. In advanced GC, the long-standing cornerstone of treatment is systemic chemotherapy [2]. However, despite recent progress in GC treatment, the dire prognosis of this cancer requires urgent improvement.
A very heterogeneous tumor, GC exhibits diverse prognoses according to various intrinsic characteristics. Therefore, the development of efficient treatment strategies for the various prognostic groups within GC is important. With this, we can more readily understand the underlying biological mechanisms of each subtype of GC to effectively individualize each treatment strategy.
Several prognostic factors in GC have been reported: performance status, tumor burden, tumor markers such as carbohydrate antigen 19-9 (CA-19-9), the high metabolic landscape of the tumor, and weight loss during chemotherapy have been independently correlated with a poor prognosis [3–7].
Growing evidence suggests that not only the tumor itself but also its niche and inflammatory cytokines are important considerations when one is defining each tumor. The neutrophil–lymphocyte ratio (NLR) has been shown to be a well-validated prognostic factor in solid tumors such as those of the lung and breast, as well as hepatocellular and renal cell carcinomas [8–12]. The NLR is a well-known biomarker of cancer-associated inflammation. However, the exact underlying biological mechanisms of how the NLR contributes to a poor prognosis have not been clearly defined, and the type of immune response related to the NLR remains unknown [13, 14].
Cytokines and angiogenic factors (CAFs) work in the context of a complex network. In cancer and especially in GC, however, an association between a simple NLR and complex CAFs has not previously been reported. To evaluate gross pathophysiological changes, along with the NLR, in cancer, CAFs associated with the NLR need to be assessed. Since a high NLR status may be caused by granulocyte recruitment and a relative lymphopenia, CAFs, which activate granulocytes and regulate lymphocytes, could be associated with the NLR [13]. Previous reports have suggested an association between the NLR and a CAF such as interleukin (IL)-17 in hepatocellular carcinoma [15], as well as IL-6, IL-8, and other CAFs in colorectal carcinoma [16, 17]. However, the demonstration of a CAF association with the NLR in GC, and the prognostic impact of such an association, is lacking. It is conceivable that a subset of CAFs could induce an immunosuppressive tumor microenvironment while at the same time also generating a premetastatic niche to promote cancer metastasis [18, 19]. Therefore, a specific subset of CAFs could have a genuine role in enhancing cancer progression.
In the current study, we aimed to evaluate the prognostic role of the NLR in the context of associated CAFs and ascertain an independent CAF signature, which can define different prognostic subsets of GC patients in addition to the NLR.
Patients and methods
Patients
This study was a retrospective analysis of deidentified patient-level data collected from medical records. Patients with GC diagnosed at Seoul National University Hospital, Republic of Korea, between September 2004 to March 2014 were included in the analysis if they were older than 18 years, had a histologically confirmed, recurrent, or metastatic GC, an Eastern Cooperative Oncology Group performance status of between 0 and 2 [20], adequate organ function, and available NLR data acquired before the administration of first-line palliative chemotherapy (NLR cohort, N = 745). The NLR was calculated as a neutrophil count divided by a lymphocyte count. During that period, we prospectively performed the biomarker study, which was approved by the Institutional Review Board, and after obtaining informed consent from patients, we collected serum samples from recurrent or metastatic GC patients before initiation of the first-line palliative chemotherapy. Among the patients who gave their consent for their participation in the biomarker study, we randomly selected 70 patients for CAF analysis in an unbiased manner (CAF cohort, N = 70).
Sample preparation and CAF analysis
All selected patients provided written informed consent for the collection of blood samples for biomarker analysis. Specimens were obtained before the initiation of palliative chemotherapy. A total of 52 CAFs present in serum were analyzed with multiplex bead suspension array kits by means of the Bio-Plex 200 system according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA), including human group I and group II cytokine panels as described in previous reports [21, 22]. Serum concentrations of soluble carbonic anhydrase IX, soluble vascular endothelial growth factor receptor 2, placental growth factor, and osteopontin were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). Each serum sample was analyzed in duplicate, and mean CAF concentrations were reported in picograms per milliliter. Some analytes, for which more than 50 % of patients had nondetectable levels or showed coefficients of variation greater than 20 %, were not included in subsequent analyses. Analytes that had nondetectable levels were recorded as half of the lower threshold value.
Statistical analysis
The primary objective of this study was to assess the prognostic impact of the NLR, and to identify CAFs that show an association with the NLR and their pragmatic implications for GC. CAF concentrations analyzed in the study were log-transformed because the concentrations were highly skewed in all samples. Overall survival (OS) and progression-free survival (PFS) were calculated as the time between the first administration of palliative first-line chemotherapy and either death or the final follow-up visit, and the time between the first administration of palliative first-line chemotherapy and when disease progression was confirmed by an imaging modality respectively. The overall response rate was defined as the proportion of patients who showed a complete or partial response, and the disease control rate was defined as the sum of complete responses, partial responses, and stable disease. Treatment responses were assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 [23].
Pearson’s chi-square test or Fisher’s exact test for any number in a group of less than 10 were performed to analyze categorical variables, including clinical characteristics. The Wilcoxon rank sum test or the Kruskal–Wallis equality-of-populations rank test in the case of two groups or more than three groups, respectively, was applied to continuous parameters. All P values were two-sided, and P < 0.05 was considered statistically significant. Additionally, in linear regression analysis to extract significant CAFs which were associated with the NLR, a false discovery rate of less than 0.05 was applied to exclude false positive correlation. Analyses were performed with use of STATA version 12 (StataCorp, College Station, TX, USA) and R version 3.1.3 (http://www.r-project.org).
Ethics
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (H-1306-007-493, H-1411-022-623). The study was conducted according to guidelines for biomedical research outlined in the Declaration of Helsinki.
Results
Patient characteristics and survival according to the NLR
A total of 745 GC patients were included for NLR analysis. Table 1 summarizes patient characteristics according to the NLR, divided into two groups according to its median value of 2.42. Patients with a high NLR had a poor performance status, and a higher tumor burden as typified by high carcinoembryonic antigen and CA-19-9 tumor marker levels and increased number of metastatic organs. Most patients received fluoropyrimidine plus platinum combination chemotherapy (capecitabine plus cisplatin, capecitabine plus oxaliplatin, leucovorin plus 5-fluorouracil plus oxaliplatin, S-1 plus cisplatin, etc.). In the case of human epidermal growth factor receptor 2 positive GC patients positive, trastzumab plus capecitabine plus cisplatin was used after the introduction of trastuzumab.
Among the 745 patients, 393 (52.8 %) were treated with second-line palliative chemotherapy after progression after first-line palliative chemotherapy. Among the 393 patients who received second-line palliative chemotherapy, 169 patients (43.0 %) received irinotecan-based hemotherapy and 115 patients (29.3 %) received taxane-based chemotherapy. One hundred ninety-nine patients (26.7 %) were treated with more than third-line palliative chemotherapy.
The median follow-up duration was 37.8 months (range 1.6–117 months). The median OS and PFS after first-line palliative chemotherapy were 14.4 months [95 % confidence interval (CI) 13.2–15.3 months] and 6.5 months (95 % CI 6.1–6.9 months) respectively. Patients in the high NLR group showed significantly worse OS and PFS [median OS 17.8 months (95 % CI 16.1–20 months) for the low NLR group vs 11.2 months (95 % CI 9.7–12.2 months) for the high NLR group, P < 0.001; median PFS 7.3 months (95 % CI 6.8–8.3 months) for the low NLR group vs 5.6 months (95 % CI 5.1–6.2 months) for the high NLR group, P < 0.001; Figs. 1a and S1a respectively]. The overall response rate was not significantly different according to the NLR ( 34.4 % for the low NLR group vs 34.8 % for the high NLR group, P = 0.850). However, the disease control rate was significantly lower in the high NLR group (80.1 % vs 86.2 %, P = 0.030).
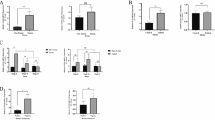
Overall survival according to the neutrophil–lymphocyte ratio (NLR). Kaplan–Meier curves (a) and forest plot of hazard ratios and 95 % confidence intervals assessed by subgroup factors (b) for overall survival of two groups classified according to a NLR higher or lower than the median value. CA-19-9 carbohydrate antigen 19-9, CEA carcinoembryonic antigen, CI confidence interval, CR complete remission, ECOG Eastern Cooperative Oncology Group performance status, HER2 human epidermal growth factor receptor 2, HR hazard ratio, mOS median overall survival, PD progressive disease, PR partial response, SD stable disease
The poor prognostic implication of the NLR was further analyzed with other clinicopathologic features. Univariate and multivariate analysis of OS and PFS showed that an NLR higher than a median value of 2.42 was significantly associated with poor survival, even after adjustment for other significant clinicopathologic features such as performance status, trastuzumab treatment, a high level of CA-19-9, the number of metastatic organs, and treatment response (P < 0.001 for OS and PFS; Tables 2 and S1 respectively). Subgroup analysis of OS and PFS showed the NLR conferred a significantly poor prognosis according to subgroups divided by each clinicopathologic feature (Figs. 1b and S1b respectively).
Significant CAFs associated with the NLR
Among the 745 patients, 70 were further analyzed in CAF analysis for an association with NLR. The clinicopathologic characteristics of the patients included in the CAF analysis are summarized in Table S2. Forty-two of 52 CAFs were subsequently analyzed, after ten CAFs had been excluded because of more than half of the samples being outside of the detection range of the assays. The mean concentrations, standard error, median concentrations, and range of concentrations of the 52 CAFs are listed in Table S3.
The association between the NLR and each CAF was analyzed by linear regression. With use of a stringent filtering condition of a false discovery rate of less than 0.05, osteopontin and IL-6 were found to be significantly associated with the NLR (Fig. 2). The associations between the NLR and all CAFs are listed in Table S4. Except for osteopontin and IL-6, melanoma growth stimulating activity α (also known as CXCL1), IL-2 receptor α, IL-12p70, and basic fibroblast growth factor were also associated with the NLR when a loose filtering condition of P < 0.05 was used.
Significant correlation of osteopontin (OPN) and interleukin-6 (IL-6) with the neutrophil–lymphocyte ratio (NLR). Scatter plot and fitted line for OPN (a) and IL-6 (b) with the NLR. R 2, the coefficient, P, and the false discovery rate (FDR) of significant cytokines and angiogenic factors that correlate with the NLR are shown (c). bFGF basic fibroblast growth factor, GRO-a melanoma growth stimulating activity α, IL-12Ra interleukin-12 receptor α, IL-12p70 interleukin-12 p70
The prognostic implication of CAFs in addition to the NLR
In addition to the NLR, some CAFs such as osteopontin and IL-6 have also been reported to be associated with a poor prognosis in GC [24, 25]. Therefore, we hypothesized that a certain subset of CAFs would have a role in the poor prognosis of GC, independently of the NLR. A survival analysis of each CAF group divided by its median value was performed, with or without adjustment for the NLR. Six CAFs—that is, stromal-cell-derived factor 1 (SDF-1), osteopontin, IL-2 receptor α, IL-8, hepatocyte growth factor, and IL-6—were significantly associated with a poor OS (Table S5). Intriguingly, the prognostic impact of SDF-1 was significant, even after adjustment for the NLR, implying that SDF-1 effected poor OS independently of the NLR. However, other CAFs, including osteopontin and IL-6, lost their prognostic significance when adjustment was made for the NLR, implying that the poor prognostic effects of other CAFs might interact with the NLR. Multivariate Cox regression analysis showed the NLR and SDF-1 were independently associated with poor survival (P = 0.002 and P = 0.036 respectively, Table 3).
A survival analysis of subgroups considering both the NLR and the SDF-1 concentration by their median values revealed that the low NLR/low SDF-1 concentration group showed the most favorable prognosis, in contrast to the high NLR/high SDF-1 concentration group, which displayed the worst OS (median OS 21.0 months for the low NLR and low SDF-1 concentration group, 15.8 months for the group with either high NLR or high SDF-1 concentration, 8.2 months for the high NLR and high SDF-1 concentration group; P = 0.229 and P = 0.003 respectively vs both low NLR and low SDF-1 concentration; Fig. 3). These trends and significances were reproduced in PFS analyses (Fig. S2). The clinicopathologic characteristics of the three groups according to the NLR and the SDF-1 concentration were not significantly different (Table S6).
Survival analysis according to the neutrophil–lymphocyte ratio (NLR) and stromal-cell-derived factor 1 (SDF-1) concentration. Kaplan–Meier curves for overall survival of three groups classified according to NLR and SDF-1 median values. CI confidential interval, HR hazard ratio, mOS median overall survival, Ref reference
Discussion
In this study, we showed that the NLR is a significant factor indicating a poor prognosis in GC. Multiplex CAF array analysis showed that the NLR was strongly associated with high serum osteopontin and IL-6 levels. In addition, a high level of SDF-1 was also independently associated with a negative impact on survival. These results suggest that both the NLR and a high serum SDF-1 level could define a poor prognostic subset of GC patients.
A close association between cancer progression and cancer-associated inflammation has been well described [14]. Among the clinically available and simple biomarkers, the NLR is one of the most reliable prognostic factors across many tumor types [13]. Although a recent report implicated the NLR in the prognosis of advanced GC [9], this is subject to independent external validation since a relatively limited number of patients (268) were included in the previous study. In the current study, we showed the clear prognostic impact of the NLR in 745 advanced GC patients, independently of other known prognostic factors such as performance status, tumor burden, CA-19-9 level, and treatment response to first-line palliative chemotherapy.
We focused on the underlying biological mechanisms implicated in the NLR conferring a poor prognosis in GC, and which subset of CAFs would be associated with the NLR, which have been poorly understood to date. A few previous studies showed that several CAFs, such as IL-6, IL-8, and IL-17, were associated with the NLR in colorectal cancer and hepatocellular carcinoma [15–17], although this association has not been reported in GC. Moreover, to the best of our knowledge, a direct association of osteopontin with the NLR has not previously been addressed in human studies. Osteopontin (also known as secreted phosphoprotein 1) and IL-6 are well-known chemotactic factors for neutrophils [26, 27]. Patients with high levels of osteopontin and IL-6 have been reported to have a poor prognosis for most tumor types, including GC [24, 28, 29]. Osteopontin can modulate extracellular remodeling to promote epithelial-mesenchymal transition and angiogenesis [30, 31]. IL-6 can activate the signal transducer and activator of transcription (STAT) signaling pathway, which induces cancer progression [27, 29]. Results from the current study showed osteopontin and IL-6 were strongly associated with the NLR, and univariate survival analysis also showed poor survival in patients with high osteopontin or high IL-6 levels.
In this study, we also found that SDF-1 was an independent prognostic factor in addition to the NLR. The interesting scope of the activities of SDF-1 regarding homeostasis of hematopoietic stem and progenitor cells in cancer induced by this chemokine has been highlighted by others [32, 33]. SDF-1 (also known as CXCL12) has an important role in maintaining leukocyte homeostasis, especially for myeloid-derived suppressor cells, which includes trafficking and directing the migration of myeloid-derived suppressor cells to immune-privileged organs or tumor microenvironments [34–36]. Since the presence of myeloid-derived suppressor cells could lower antitumor immunity in a tumor microenvironment [37], the recruitment of such cells induced by SDF-1 would allow cancer progression, which supports our findings of SDF-1 as a factor in the poor prognosis of GC. Previous reports have also shown tumor expression or peripheral levels of SDF-1 were related to poor prognoses in ovarian and colon cancers [38, 39]. This current study has highlighted the prognostic impact of serum SDF-1 in GC for the first time.
Although the prognostic impact of the NLR was validated in a large number of patients in the current study (N = 745), analysis of the association of CAFs with the NLR was performed in a relatively small number of patients (N = 70). Among the patients who gave their consent for their participation in the biomarker study, we could not analyze all the samples because of limited amounts of remaining samples. Therefore, we randomly selected only 70 patients for CAF analysis. Even though this CAF cohort was randomly selected in an unbiased manner, the retrospective analysis design per se limits generalization of the findings. To interpret the CAF analysis, the other factors, such as use of steroid, which could modulate such signals should also be considered. Such information is limited in the retrospective design study. Similarly, the changes in supportive care during the 10-year study period are to be considered for the accurate interpretation of survival outcomes, even though supportive care has been changed in our hospital. Among the 52 CAFs initially planned to be analyzed, ten were excluded because of more than half of the samples being outside the detection range of assays. It is very difficult to explain why these ten CAFs were not detected well enough. A possible explanation might include the amount of the CAF itself, the sensitivity of the detection method, the stability of the CAF, the differences in tumor types, etc.
Despite these caveats, the multiplex analysis of CAFs for evaluation of any association with the NLR is reported in GC for the first time, and the finding of an association between osteopontin and the NLR is revealed. Moreover, the prognostic significance of SDF-1, especially independently of the NLR, is raised in the current study.
From the findings taken together, the NLR is a significant factor for a poor prognosis in advanced GC. The poor prognostic impact of the NLR may be related to the biological actions of osteopontin and IL-6. As well as the NLR, a high level of SDF-1 independently had a negative impact on survival. Therefore, consideration of both the NLR and serum SDF-1 could define a poor prognostic subset of GC patients, highlighting these might provide insights into antitumor immunity in GC. What kind of innovative treatment is essential in this poor prognostic subset of GC is still an open challenging question. One of the strategies might be to harness immuno-oncology drugs to modulate these antitumor immunities.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;(3):CD004064.
Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–403.
Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94:637–41.
Jo JC, Ryu MH, Koo DH, Ryoo BY, Kim HJ, Kim TW, et al. Serum CA 19-9 as a prognostic factor in patients with metastatic gastric cancer. Asia Pac J Clin Oncol. 2013;324–30
Ock CY, Kim TY, Lee KH, Han SW, Im SA, Kim TY, et al. Metabolic landscape of advanced gastric cancer according to HER2 and its prognostic implications. Gastric Cancer. 2016;19;420–30.
Ock CY, Oh DY, Lee J, Kim TY, Lee KH, Han SW, et al. Weight loss at the first month of palliative chemotherapy predicts survival outcomes in patients with advanced gastric cancer. Gastric Cancer. 2016;19;597–606.
Zhang X, Zhang W, Feng LJ. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One. 2014;9:e111906.
Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17:703–10.
Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24.
Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117.
Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015;5:e006404.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64.
Kantola T, Klintrup K, Vayrynen JP, Vornanen J, Bloigu R, Karhu T, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729–36.
Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088–97.
Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31.
Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–64.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Montero AJ, Diaz-Montero CM, Millikan RE, Liu J, Do KA, Hodges S, et al. Cytokines and angiogenic factors in patients with metastatic renal cell carcinoma treated with interferon-alpha: association of pretreatment serum levels with survival. Ann Oncol. 2009;20:1682–7.
Zurita AJ, Jonasch E, Wang X, Khajavi M, Yan S, Du DZ, et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:46–52.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–9.
Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155.
Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–45.
Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–24.
Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–81.
Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol. 2014;89:129–39.
Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61.
Ali S, Lazennec G. Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007;26:401–20.
Guo F, Wang Y, Liu J, Mok SC, Xue F, and Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35:816–26.
Karin N. The multiple faces of CXCL12 (SDF-1α) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88:463–73.
Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE2-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70.
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Popple A, Durrant LG, Spendlove I, Rolland P, Scott IV, Deen S, et al. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br J Cancer. 2012;106:1306–13.
Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, de Braud F, Gevorgyan A, et al. Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens. Cancers (Basel). 2014;6:1753–68.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (grant no. 2013R1A1A2008705) and also by a grant from the Seoul National University College of Medicine (800-20140609).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for their being included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ock, CY., Nam, AR., Lee, J. et al. Prognostic implication of antitumor immunity measured by the neutrophil–lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer 20, 254–262 (2017). https://doi.org/10.1007/s10120-016-0613-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-016-0613-5