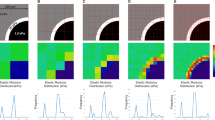
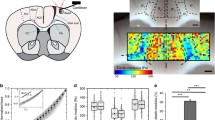
Abstract
Although glia have been historically classified as the structurally supporting cells of the central nervous system, their role in tissue mechanics is still largely unstudied. The influence of myelin and glia on the mechanical properties of spinal cord tissue was examined by testing embryonic day 18 chick embryo spinal cords in uniaxial tension following disruption of the glial matrix using either ethidium bromide (EB) or an antibody against galactocerebroside (αGalC) in the presence of complement. Demyelination was confirmed by myelin basic protein immunoreactivity and quantified using osmium tetroxide staining. A substantial loss of astrocytes and oligodendrocytes concurrent with demyelination was observed following EB injection but not αGalC injection. No morphological changes were observed following injection of saline or IgG with complement as controls for EB and αGalC. Demyelinated spinal cords demonstrated significantly lower stiffness and ultimate tensile stress than myelinated spinal cords. No significant differences were observed in the tensile response between the two demyelinating protocols. The results demonstrate that the glial matrix provides significant mechanical support to the spinal cord, and suggests that myelin and cellular coupling of axons via the glial matrix in large part dictates the tensile response of the tissue.
Similar content being viewed by others
References
Arbogast KB, Margulies SS (1999) A fiber-reinforced composite model of the viscoelastic behavior of the brainstem in shear. J Biomech 32(8): 865–870. doi:10.1016/S0021-9290(99)00042-1
Bain AC, Meaney DF (2000) Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J Biomech Eng 122(6): 615–622. doi:10.1115/1.1324667
Bain AC, Shreiber DI, Meaney DF (2003) Modeling of microstructural kinematics during simple elongation of central nervous system tissue. J Biomech Eng 125(6): 798–804. doi:10.1115/1.1632627
Balgude AP, Yu X, Szymanski A, Bellamkonda RV (2001) Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 22(10): 1077–1084. doi:10.1016/S0142-9612(00)00350-1
Bilston LE, Thibault LE (1996) The mechanical properties of the human cervical spinal cord in vitro. Ann Biomed Eng 24(1): 67–74. doi:10.1007/BF02770996
Borschel GH, Kia KF, Kuzon WM Jr, Dennis RG (2003) Mechanical properties of acellular peripheral nerve. J Surg Res 114(2): 133–139. doi:10.1016/S0022-4804(03)00255-5
Butt AM, Colquhoun K, Tutton M, Berry M (1994) Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve. J Neurocytol 23(8): 469–485. doi:10.1007/BF01184071
Bylski DI, Kriewall TJ, Akkas N, Melvin JW (1986) Mechanical behavior of fetal dura mater under large deformation biaxial tension. J Biomech 19(1): 19–26. doi:10.1016/0021-9290(86)90105-3
Carroll WM, Jennings AR, Mastaglia FL (1987) Reactive glial cells in CNS demyelination contain both GC and GFAP. Brain Res 411(2): 364–369. doi:10.1016/0006-8993(87)91088-2
Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N et al (2002) Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol 12(3): 217–220. doi:10.1016/S0960-9822(01)00680-7
Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751): 1139–1143. doi:10.1126/science.1116995
Dyer CA, Benjamins JA (1990) Glycolipids and transmembrane signaling: antibodies to galactocerebroside cause an influx of calcium in oligodendrocytes. J Cell Biol 111(2): 625–633. doi:10.1083/jcb.111.2.625
Elkin BS, Azeloglu EU, Costa KD, Morrison B III (2007) Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma 24(5): 812–822. doi:10.1089/neu.2006.0169
Fernandes CG, Graca DL, Pereira LA (1998) Inflammatory response of the spinal cord to multiple episodes of blood–brain barrier disruption and toxic demyelination in Wistar rats. Braz J Med Biol Res 31(7): 933–936. doi:10.1590/S0100-879X1998000700008
Fitzner D, Schneider A, Kippert A, Mobius W, Willig KI, Hell SW et al (2006) Myelin basic protein-dependent plasma membrane reorganization in the formation of myelin. EMBO J 25(21): 5037–5048. doi:10.1038/sj.emboj.7601376
Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA (2002) Neurite branching on deformable substrates. Neuroreport 13(18): 2411–2415. doi:10.1097/00001756-200212200-00007
Gefen A, Gefen N, Linder-Ganz E, Margulies SS (2005) In vivo muscle stiffening under bone compression promotes deep pressure sores. J Biomech Eng 127(3): 512–524. doi:10.1115/1.1894386
Gennarelli TA, Tipperman R, Maxwell WL, Graham DI, Adams JH, Irvine A (1993) Traumatic damage to the nodal axolemma: an early, secondary injury. Acta Neurochir Suppl (Wien) 57: 49–52
Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA (2006) Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90(8): 3012–3018. doi:10.1529/biophysj.105.073114
Graca DL, Blakemore WF (1986) Delayed remyelination in rat spinal cord following ethidium bromide injection. Neuropathol Appl Neurobiol 12(6): 593–605. doi:10.1111/j.1365-2990.1986.tb00162.x
Hao H, Shreiber DI (2007) Axon kinematics change during growth and development. J Biomech Eng 129(4): 511. doi:10.1115/1.2746372
Heidemann SR, Buxbaum RE (1994) Mechanical tension as a regulator of axonal development. Neurotoxicology 15(1): 95–107
Heredia A, Bui CC, Suter U, Young P, Schaffer TE (2007) AFM combines functional and morphological analysis of peripheral myelinated and demyelinated nerve fibers. Neuroimage 37(4): 1218–1226. doi:10.1016/j.neuroimage.2007.06.007
Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A et al (2005) Nectin-like molecule-1/TSLL1/ SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J Cell Sci 118(Pt 6): 1267–1277. doi:10.1242/jcs.01656
Keirstead HS, Hasan SJ, Muir GD, Steeves JD (1992) Suppression of the onset of myelination extends the permissive period for the functional repair of embryonic spinal cord. Proc Natl Acad Sci USA 89(24): 11664–11668. doi:10.1073/pnas.89.24.11664
Keirstead HS, Pataky DM, McGraw J, Steeves JD (1997) In vivo immunological suppression of spinal cord myelin development. Brain Res Bull 44(6): 727–734. doi:10.1016/S0361-9230(97)00374-2
Lu YB, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolburg H et al (2006) Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA 103(47): 17759–17764. doi:10.1073/pnas.0606150103
Mastaglia FL, Carroll WM, Jennings AR (1989) Spinal cord lesions induced by antigalactocerebroside serum. Clin Exp Neurol 26: 33–44
Meaney DF (2003) Relationship between structural modeling and hyperelastic material behavior: application to CNS white matter. Biomech Model Mechanobiol 1(4): 279–293. doi:10.1007/s10237-002-0020-1
Meaney DF, Margulies SS, Smith DH (2001) Diffuse axonal injury. J Neurosurg 95(6): 1108–1110
Mueller H, Butt HJ, Bamberg E (1999) Force measurements on myelin basic protein adsorbed to mica and lipid bilayer surfaces done with the atomic force microscope. Biophys J 76(2): 1072–1079
Ozawa K, Saida T, Saida K, Nishitani H, Kameyama M (1989) In vivo CNS demyelination mediated by anti-galactocerebroside antibody. Acta Neuropathol 77(6): 621–628. doi:10.1007/BF00687890
Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R et al (1997) Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J 16(5): 978–988. doi:10.1093/emboj/16.5.978
Pereira LA, Dertkigil MS, Graca DL, Cruz-Hofling MA (1998) Dynamics of remyelination in the brain of adult rats after exposure to ethidium bromide. J Submicrosc Cytol Pathol 30(3): 341–348
Reynolds R, di Bello IC, Meeson A, Piddlesden S (1996) Comparison of a Chemically Mediated and an Immunologically Mediated Demyelinating Lesion Model. Methods 10(3): 440–452. doi:10.1006/meth.1996.0122
Schultze M (1866) Anatomie und Physiologe der Retina. Max Cohen & Sons, Bonn
Sergott RC, Brown MJ, Silberberg DH, Lisak RP (1984) Antigalactocerebroside serum demyelinates optic nerve in vivo. J Neurol Sci 64(3): 297–303. doi:10.1016/0022-510X(84)90177-1
Sherman DL, Brophy PJ (2005) Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6(9): 683–690. doi:10.1038/nrn1743
(1856) Abhandlungen zur Wissenschafilichen medicin. Meidinger Sohn, Frankfurt
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6(8): 626–640. doi:10.1038/nrn1722
Yuan Q, Dougherty L, Margulies SS (1998) In vivo human cervical spinal cord deformation and displacement in flexion. Spine 23(15): 1677–1683. doi:10.1097/00007632-199808010-00012
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shreiber, D.I., Hao, H. & Elias, R.A. Probing the influence of myelin and glia on the tensile properties of the spinal cord. Biomech Model Mechanobiol 8, 311–321 (2009). https://doi.org/10.1007/s10237-008-0137-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-008-0137-y