Abstract
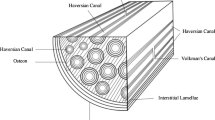
The mechanism by which mechanical stimulation on osteocytes results in biochemical signals that initiate the remodeling process inside living bone tissue is largely unknown. Even the type of stimulation acting on these cells is not yet clearly identified. However, the cytoskeleton of osteocytes is suggested to play a major role in the mechanosensory process due to the direct connection to the nucleus. In this paper, a computational approach to model and simulate the cell structure of osteocytes based on self-stabilizing tensegrity structures is suggested. The computational model of the cell consists of the major components with respect to mechanical aspects: the integrins that connect the cell with the extracellular bone matrix, and different types of protein fibers (microtubules and intermediate filaments) that form the cytoskeleton, the membrane-cytoskeleton (microfilaments), the nucleus and the centrosome. The proposed geometrical cell models represent the cell in its physiological environment which is necessary in order to give a statement on the cell behavior in vivo. Studies on the mechanical response of osteocytes after physiological loading and in particular the mechanical response of the nucleus show that the load acting on the nucleus is rising with increasing deformation applied to the integrins.
Similar content being viewed by others
References
Balzani D, Schröder J, Gross D (2006) Simulation of discontinuous damage incorporating residual stresses in circumferentially overstreched atherosclerotic arteries. Acta Biomaterialia 2: 609–618
Balzani D, Schröder J, Gross D (2007) Numerical simulation of residual stresses in arterial walls. Comput Mater Sci 39: 117–123
Baudriller H, Maurin B, Nadas PC, Montcourrier P, Parmeggiani A, Bettache N (2006) Form-finding of complex tensegrity structures: application to cell cytoskeleton modelling. Comptes Rendus Mécanique 334: 662–668
Benninghoff A, Drenckhahn D (2008) Anatomie. Makroskopische Anatomie, Histologie, Embryologie, Zellbiologie, Bd. 1. Elsevier (Urban & Fischer), München/Jena
Beno T, Yoon YJ, Cowin SC, Fritton SP (2006) Estimation of bone permeability using accurate microstructural measurements. J Biomech 39: 2378–2387
Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB (1998) Intracortical remodeling in adult rat long bones after fatigue loading. Bone 23: 275–281
Bonivtch AR, Bonewald LF, Nicolella DP (2007) Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech 40: 2199–2206
Burger EH, Klein-Nulend J (1999) Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J 13: S101–S112
Burr DB, Martin RB, Schaffler MB, Radin EL (1985) Bone remodeling in response to in-vivo fatigue microdamage. J Biomech 18: 189–200
Caille N, Thoumine O, Tardy Y, Meister JJ (2002) Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35: 177–187
Chen CS, Ingber DE (2008) Tensegrity und mechanoregulation: vom skelett zum zytoskelett. Osteopathische Medizin, Zeitschrift für ganzheitliche Heilverfahren 9: 4–17
Cowin, SC (ed) (2001) Bone mechanics handbook. CRC Press, Boca Raton
Cowin SC (2007) The significance of bone microstructure in mechanotransduction. J Biomech 40: S105–S109
Cowin SC, Doty SB (2006) Tissue mechanics. Springer, New York
Currey JD (2002) Bones: structure and mechanics. Princeton University Press, Princeton
Deriu MA, Enemark S, Soncini M, Montevecchi FM, Redaelli A (2007) Tubulin: from atomistic structure to supramolecular mechanical properties. J Mater Sci 42: 2007
Frost HM (1960) Presence of microscopic cracks in vivo in bone. Bull Henry Ford Hosp 8: 25–35
Frost HM (1985) Bone microdamage: Factors that impair its repair. In: Uhthoff HK (ed) Current concepts in bone fragility. Springer, Berlin, pp 123–148
Fudge DS, Gardner KH, Forsyth VT, Riekel C, Gosline JM (2003) The mechanical properties of hydrated intermediate filaments: insights from hagfish slime threads. Biophys J 85: 2015–2027
Gittes F, Mickey B, Nettleton J, Howard J (1993) Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol 120: 923–934
Ingber DE (1993) Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104: 613–627
Ingber DE (1997) Tensgrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575–599
Ingber DE (2003) Tensegrity i. cell structure and hierarchical systems biology. J Cell Sci 116: 1157–1173
Janmey PA, Euteneuer U, Traub P, Schliwa M (1991) Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol 113: 155–160
Kahla NB, Kebiche K (2000) Nonlinear elastoplastic analysis of tensegrity systems. Eng Struct 23: 1552–1566
Karcher H, Lammerding J, Huang H, Lee RT, Kamm RD, Kaazempur-Mofrad MR (2003) A three-dimensional viscoelastic model for cell deformation with experimental verification. Biophys J 85: 3336–3349
Kardas D (2010) A multiscale computational approach for microcrack evolution in cortical bone and related mechanical stimulation of bone cells. PhD thesis, Institut für Baumechanik und Numerische Mechanik, Leibniz Universität Hannover
Kebiche K, Kazi-Aoual M, Motro R (1999) Geometrical non-linear analysis of tensegrity systems. Eng Struct 21: 864–876
Lenz C (2005) Numerical micro-meso modelling of mechanosensation driven osteonal remodelling in cortical bone. PhD thesis, Institut für Baumechanik und Numerische Mechanik, Leibniz Universität Hannover
Maejuma-Ikeda A, Aoki M, Tsuritani K, Kamioka K, Hiura K, Miyoshi T, Hara H, Takano-Yamamoto T, Kumegawa M (1997) Chick osteocyte-derived protein inhibits osteoclastic bone resorption. Biochem J 322: 245–250
Marotti G, Ferretti M, Muglia MA, Palumbo C, Palazzini S (1992) A quantitative evaluation of osteoblast-osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone 13: 363–368
Martin RB (2000) Toward a unifying theory of bone remodeling. Bone 26: 1–6
Martin RB (2002) Is all cortical bone remodeling initiated by microdamage?. Bone 30: 8–13
Martin RB, Burr DB (1982) A hypothetical mechanism for the stimulation of osteonal remodelling by fatigue damage. J Biomech 15: 137–139
Martin RB, Burr DB, Sharkey NA (1998) Skeletal tissue mechanics. Springer, Berlin
Maurin B, nadas PC, Baudriller H, Montcourrier P, Bettache N (2008) Mechanical model of cytoskeleton structuration during cell adhesion and spreading. J Biomech 41: 2036–2041
McCreadie BR, Hollister SJ, Schaffler MB, Goldstein SA (2003) Osteocyte lacuna size and shape in women with and without osteoporotic fracture. J Biomech 37: 563–572
McGarry JG, Prendergast P (2004) A three-dimensional finite element model of an adherent eukaryotic cell. Eur Cells Mater 7: 27–34
McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ (2005) A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J 19: 482–484
Mehrbod M, Mofrad MRK (2011) On the significance of microtubule flexural behavior in cytoskeletal mechanics. PLoS one 6: 1–10
Mullender M, Haj AE, Yang Y, Duin M, van Burger EH, Klein-Nulend J (2004) Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput 42: 14–21
Murakami H (2001) Static and dynamic analyses of tensegrity structures. Part 1. Nonlinear equations of motion. Int J Solids Struct 38: 3599–3613
Nackenhorst U, Lenz C (2005) Biomechanics of bones on various length scales. Proc Appl Math Mech 5: 31–34
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J (2006) Osteocyte lacunae tissue strain in cortical bone. J Biomech 39: 1735–1743
Nicolella PD, Lankford J (2002) Microstructural strain near osteocyte lacuna in cortical bone in vitro. J Musculoskelet Neuron Interact 2: 261–263
Norman TL, Wang Z (1997) Microdamage of human cortical bone: incidence and morphology in long bones. Bone 20: 375–379
Palmer JS (2008) Microstructurally-based constitutive models of cytoskeletal networks for simulation of the biomechanical response of biological cells. PhD thesis, Department of Mechanical Engineering, Massachusetts Institute of Technology
Prendergast PJ, Huiskes R (1996) Microdamage and osteocyte-lacuna strain in bone: a microstructural finite element analysis. J Biomech Eng 118: 240–246
Shafrir Y, Forgacs G (2002) Mechanotransduction through the cytoskeleton. Am J Physiol Cell Physiol 282: C479–C486. doi:10.1152/ajpcell.00394.2001
Sissons HA, O’Connor P (1977) Quantitative histology of osteocyte lacunae in normal human cortical bone. Calcif Tissue Res 22: 530–533
Stamenovic D, Fredberg J, Wang N, Butler JP, Ingber DE (1996) A microstructural approach to cytoskeletal mechanics based on tensegrity. J Theor Biol 181: 125–136
Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS (1998) Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res 1998: 1555–1568
Vatsa A, Smit TH, Klein-Nulend J (2007) Extracellular no signalling from a mechanically stimulated osteocyte. J Biomech 40: S89–S95
Vogel V (2006) Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct 35: 459–488. doi:10.1146/annurev.biophys.35.040405.102013
Wang N, Stamenovic D (2000) Contribution of intermediate filaments to cell stiffness, stiffening and growth. Am J Physiol 279: C188–C194
Wang N, Naruse K, Stamenović D, Fredberg JJ, Mijailovich SM, Tolić-Norrelykke IM, Polte T, Mannix R, Ingber DE (2001) Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci USA 98: 7765–7770
Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27: 339–360
You L, Cowin SC, Schaffler MB, Weinbaum S (2001) A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech 34: 1375–1386
Zhang J, Ohsaki M (2006) Adaptive force density method for form-finding problem of tensegrity structures. Int J Solids Struct 43: 5658–5673
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kardas, D., Nackenhorst, U. & Balzani, D. Computational model for the cell-mechanical response of the osteocyte cytoskeleton based on self-stabilizing tensegrity structures. Biomech Model Mechanobiol 12, 167–183 (2013). https://doi.org/10.1007/s10237-012-0390-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-012-0390-y