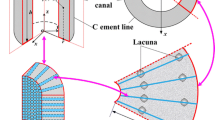
Abstract
Osteocytes are believed to be the primary sensor of mechanical stimuli in bone, which orchestrate osteoblasts and osteoclasts to adapt bone structure and composition to meet physiological loading demands. Experimental studies to quantify the mechanical environment surrounding bone cells are challenging, and as such, computational and theoretical approaches have modelled either the solid or fluid environment of osteocytes to predict how these cells are stimulated in vivo. Osteocytes are an elastic cellular structure that deforms in response to the external fluid flow imposed by mechanical loading. This represents a most challenging multi-physics problem in which fluid and solid domains interact, and as such, no previous study has accounted for this complex behaviour. The objective of this study is to employ fluid–structure interaction (FSI) modelling to investigate the complex mechanical environment of osteocytes in vivo. Fluorescent staining of osteocytes was performed in order to visualise their native environment and develop geometrically accurate models of the osteocyte in vivo. By simulating loading levels representative of vigorous physiological activity (\(3,000\,\upmu \upvarepsilon \) compression and 300 Pa pressure gradient), we predict average interstitial fluid velocities \((\sim 60.5\,\upmu \text{ m/s })\) and average maximum shear stresses \((\sim 11\, \text{ Pa })\) surrounding osteocytes in vivo. Interestingly, these values occur in the canaliculi around the osteocyte cell processes and are within the range of stimuli known to stimulate osteogenic responses by osteoblastic cells in vitro. Significantly our results suggest that the greatest mechanical stimulation of the osteocyte occurs in the cell processes, which, cell culture studies have indicated, is the most mechanosensitive area of the cell. These are the first computational FSI models to simulate the complex multi-physics mechanical environment of osteocyte in vivo and provide a deeper understanding of bone mechanobiology.








Similar content being viewed by others
References
Adachi T, Aonuma Y, Tanaka M, Hojo M, Takano-Yamamoto T, Kamioka H (2009) Calcium response in single osteocytes to locally applied mechanical stimulus: differences in cell process and cell body. J Biomech 42(12):1989–1995
Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH (1996) Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes–A cytoskeleton-dependent process. Biochem Biophys Res Commun 225(1):62–68. doi:10.1006/bbrc.1996.1131
Anderson E, Kaliyamoorthy S, Alexander J, Tate M (2005) Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann Biomed Eng 33(1):52–62. doi:10.1007/s10439-005-8962-y
Anderson E, Kreuzer S, Small O (2008) Pairing computational and scaled physical models to determine permeability as a measure of cellular communication in micro- and nano-scale pericellular spaces. Microfluid Nanofluid 4(3):193–204. doi:10.1007/s10404-007-0156-5
Anderson EJ, Knothe Tate ML (2008) Idealization of pericellular fluid space geometry and dimension results in a profound underprediction of nano-microscale stresses imparted by fluid drag on osteocytes. J Biomech 41(8):1736–1746
Appelman TP, Mizrahi J, Seliktar D (2011) A finite element model of cell-matrix interactions to study the differential effect of scaffold composition on chondrogenic response to mechanical stimulation. J Biomech Eng 133(4):041010–041012
Bacabac RG, Mizuno D, Schmidt CF, MacKintosh FC, Van Loon JJWA, Klein-Nulend J, Smit TH (2008) Round versus flat: bone cell morphology, elasticity, and mechanosensing. J Biomech 41(7):1590–1598
Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJWA, Klein-Nulend J (2004) Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun 315(4):823–829
Bakker AD, Soejima K, Klein-Nulend J, Burger EH (2001) The production of nitric oxide and prostaglandin E2 by primary bone cells is shear stress dependent. J Biomech 34(5):671–677
Biot MA (1941) General theory of three-dimensional consolidation. J Appl Phys 12(2):155–164. doi:10.1063/1.1712886
Biot MA (1955) Theory of elasticity and consolidation for a porous anisotropic solid. J Appl Phys 26(2):182–185. doi:10.1063/1.1721956
Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM (2012) Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater 23:13–17
Birmingham E, Grogan JA, Niebur GL, McNamara LM, McHugh PE (2013) Computational modelling of the mechanics of trabecular bone and marrow using fluid structure interaction techniques. Ann Biomed Eng 41(4):814–826. doi:10.1007/s10439-012-0714-1
Burger EH, Veldhuijzen JP (1993) Influence of mechanical factors on bone formation, resorption and growth in vitro. Bone 7:37–56
Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A (1996) In vivo measurement of human tibial strains during vigorous activity. Bone 18(5):405–410
Chatterjee N, Chatterjee A (2001) Role of alphavbeta3 integrin receptor in the invasive potential of human cervical cancer (SiHa) cells. J Environ Pathol Toxicol Oncol 20(3):211–221
Cheng JT, Giordano N (2002) Fluid flow through nanometer-scale channels. Phys Rev E 65(3):031206
Clover J, Dodds RA, Gowen M (1992) Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci 103(1):267–271
Darling EM, Topel M, Zauscher S, Vail TP, Guilak F (2008) Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J Biomech 41(2):454–464
Deligianni D, Apostolopoulos C (2008) Multilevel finite element modeling for the prediction of local cellular deformation in bone. Biomech Model Mechanobiol 7(2):151–159. doi:10.1007/s10237-007-0082-1
Devoll RE, Pinero GJ, Appelbaum ER, Dul E, Troncoso P, Butler WT, Farach-Carson MC (1997) Improved immunohistochemical staining of osteopontin (OPN) in paraffin-embedded archival bone specimens following antigen retrieval: anti-human OPN antibody recognizes multiple molecular forms. Calcif Tissue Int 60(4):380–386. doi:10.1007/s002239900247
Dowling EP, Ronan W, Ofek G, Deshpande VS, McMeeking RM, Athanasiou KA, McGarry JP (2012) The effect of remodelling and contractility of the actin cytoskeleton on the shear resistance of single cells: a computational and experimental investigation. J R Soc Interface. 9(77):3469–3479. doi:10.1098/rsif.2012.0428
Engleman VW, Nickols GA, Ross FP, Horton MA, Griggs DW, Settle SL, Ruminski PG, Teitelbaum SL (1997) A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest 99(9):2284–2292. doi:10.1172/JCI119404
Fritton SP, J. McLeod K, Rubin CT (2000) Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech 33(3):317–325
Goldstein SA, Matthews LS, Kuhn JL, Hollister SJ (1991) Trabecular bone remodeling: an experimental model. J Biomech 24:Supplement 1:135–150
Goulet G, Coombe D, Martinuzzi R, Zernicke R (2009) Poroelastic evaluation of fluid movement through the lacunocanalicular system. Ann Biomed Eng 37(7):1390–1402. doi:10.1007/s10439-009-9706-1
Gururaja S, Kim H, Swan C, Brand R, Lakes R (2005) Modeling deformation-induced fluid flow in cortical bone’s canalicular-lacunar system. Ann Biomed Eng 33(1):7–25. doi:10.1007/s10439-005-8959-6
Han Y, Cowin SC, Schaffler MB, Weinbaum S (2004) Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA 101(47):16689–16694. doi:10.1073/pnas.0407429101
Horton MA, Taylor ML, Arnett TR, Helfrich MH (1991) Arg Gly Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res 195(2):368–375. doi:10.1016/0014-4827(91)90386-9
Huang S, Stupack D, Liu A, Cheresh D, Nemerow GR (2000) Cell growth and matrix invasion of EBV-immortalized human B lymphocytes is regulated by expression of alpha(v) integrins. Oncogene 19(15):1915–1923
Kamioka H, Kameo Y, Imai Y, Bakker AD, Bacabac RG, Yamada N, Takaoka A, Yamashiro T, Adachi T, Klein-Nulend J (2012) Microscale fluid flow analysis in a human osteocyte canaliculus using a realistic high-resolution image-based three-dimensional model. Integr Biol
Kamioka H, Sugawara Y, Murshid SA, Ishihara Y, Honjo T, Takano-Yamamoto T (2006) Fluid shear stress induces less calcium response in a single primary osteocyte than in a single osteoblast: implication of different focal adhesion formation. J Bone Miner Res 21(7):1012–1021. doi:10.1359/jbmr.060408
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH (1995) Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J 9(5):441–445
Knothe Tate M (2001) Mixing mechanisms and net solute transport in bone. Ann Biomed Eng 29(9):810–811. doi:10.1114/1.1397788
Knothe Tate ML (2003) Whither flows the fluid in bone? An osteocyte’s perspective. J Biomech 36(10):1409–1424
Knothe Tate ML, Knothe U (2000) An ex vivo model to study transport processes and fluid flow in loaded bone. J Biomech 33(2):247–254. doi:10.1016/s0021-9290(99)00143-8
Knothe Tate ML, Knothe U, Niederer P (1998a) Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci 316(3):189–195
Knothe Tate ML, Niederer P (1998) A theoretical FE-base model developed to predict the relative contribution of convective and diffusive transport mechanisms for the maintenance of local equilibria within cortical bone. Paper presented at the Advances in Heat and Mass Transfer in Biotechnology, Anaheim, California
Knothe Tate ML, Niederer P, Knothe U (1998b) In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22(2):107–117. doi:10.1016/s8756-3282(97)00234-2
Knothe Tate ML, Steck R, Forwood MR, Niederer P (2000) In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol 203(18):2737–2745
Lanyon LE, Rubin CT (1984) Static versus dynamic loads as an influence on bone remodelling. J Biomech 17(12):897–905
Mak AFT, Huang DT, Zhang JD, Tong P (1997) Deformation-induced hierarchical flows and drag forces in bone canaliculi and matrix microporosity. J Biomech 30(1):11–18
Manfredini P, Cocchetti G, Maier G, Redaelli A, Montevecchi FM (1999) Poroelastic finite element analysis of a bone specimen under cyclic loading. J Biomech 32(2):135–144
McGarry J (2009) Characterization of cell mechanical properties by computational modeling of parallel plate compression. Ann Biomed Eng 37(11):2317–2325. doi:10.1007/s10439-009-9772-4
McGarry JP, Fu J, Yang MT, Chen CS, McMeeking RM, Evans AG, Deshpande VS (2009) Simulation of the contractile response of cells on an array of micro-posts. Philos Trans R Soc A Math Phys Eng Sci 367(1902):3477–3497. doi:10.1098/rsta.2009.0097
McKee MD, Nanci A (1996) Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech 33(2):141–164
McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB (2009) Attachment of osteocyte cell processes to the bone matrix. Anat Rec 292(3):355–363. doi:10.1002/ar.20869
Noda M, Tsuji K, Nifuji A (2003) Osteopontin: a topic from the point of bone morphology. Clin Calcium 13(4):464–466
Ofek G, Dowling E, Raphael R, McGarry J, Athanasiou K (2010) Biomechanics of single chondrocytes under direct shear. Biomech Model Mechanobiol 9(2):153–162. doi:10.1007/s10237-009-0166-1
Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE, Duncan RL (1997) Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol Cell Physiol 273(3):C810–C815
Piekarski K, Munro M (1977) Transport mechanism operating between blood supply and osteocytes in long bones. Nature 269(5623):80–82
Price C, Zhou X, Li W, Wang L (2011) Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J Bone Miner Res 26(2):277–285. doi:10.1002/jbmr.211
Rath Bonivtch A, Bonewald LF, Nicolella DP (2007) Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech 40(10):2199–2206. doi:10.1016/j.jbiomech.2006.10.040
Ronan W, Deshpande VS, McMeeking RM, McGarry JP (2012) Numerical investigation of the active role of the actin cytoskeleton in the compression resistance of cells. J Mech Behav Biomed Mater 14:143–157. doi:10.1016/j.jmbbm.2012.05.016
Ross FP, Chappel J, Alvarez JI, Sander D, Butler WT, Farach-Carson MC, Mintz KA, Robey PG, Teitelbaum SL, Cheresh DA (1993) Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem 268(13):9901–9907
Sansalone V, Kaiser J, Naili S, Lemaire T (2012) Interstitial fluid flow within bone canaliculi and electro-chemo-mechanical features of the canalicular milieu. Biomech Model Mechanobiol 1–21. doi:10.1007/s10237-012-0422-7
Sittichockechaiwut A, Scutt AM, Ryan AJ, Bonewald LF, Reilly GC (2009) Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone 44(5):822–829. doi:10.1016/j.bone.2008.12.027
Smalt R, Mitchell FT, Howard RL, Chambers TJ (1997) Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. American Journal of Physiology - Endocrinology And Metabolism 273(4):E751–E758
Sodek J, McKee MD (2000) Molecular and cellular biology of alveolar bone. Periodontology 24(1):99–126. doi:10.1034/j.1600-0757.2000.2240106.x
Steck R, Niederer P (2003) A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. J Theor Biol 220(2):249–259
Sugawara Y, Ando R, Kamioka H, Ishihara Y, Murshid SA, Hashimoto K, Kataoka N, Tsujioka K, Kajiya F, Yamashiro T, Takano-Yamamoto T (2008) The alteration of a mechanical property of bone cells during the process of changing from osteoblasts to osteocytes. Bone 43(1):19–24
Tanaka-Kamioka K, Kamioka H, Ris H, Lim S-S (1998) Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res 13(10):1555–1568. doi:10.1359/jbmr.1998.13.10.1555
Taylor ME, Tanner KE, Freeman MAR, Yettram AL (1996) Stress and strain distribution within the intact femur: compression or bending? Med Eng Phys 18(2):122–131. doi:10.1016/1350-4533(95)00031-3
Vatsa A, Semeins CM, Smit TH, Klein-Nulend J (2008) Paxillin localisation in osteocytes–is it determined by the direction of loading? Biochem Biophys Res Commun 377(4):1019–1024. doi:10.1016/j.bbrc.2007.12.174
Vaughan TJ, Haugh MG, McNamara LM (2013) A fluid-structure interaction model to characterize bone cell stimulation in parallel-plate flow chamber systems. J R Soc Interface 10(81). doi:10.1098/rsif.2012.0900
Vaughan TJ, McCarthy CT, McNamara LM (2012) A three-scale finite element investigation into the effects of tissue mineralisation and lamellar organisation in human cortical and trabecular bone. J Mech Behav Biomed Mater 12:50–62
Verbruggen SW, Vaughan TJ, McNamara LM (2012) Strain amplification in bone mechanobiology: a computational investigation of the in vivo mechanics of osteocytes. J R Soc Interface. doi:10.1098/rsif.2012.0286
Wang L, Cowin SC, Weinbaum S, Fritton SP (2000) Modeling tracer transport in an osteon under cyclic loading. Ann Biomed Eng 28(10):1200–1209. doi:10.1114/1.1317531
Wang L, Cowin S, Weinbaum S, Fritton S (2001) In Response to “Mixing Mechanisms and Net Solute Transport in Bone” by M. L. Knothe Tate. Ann Biomed Eng 29(9):812–816. doi:10.1114/1.1397789
Wang L, Wang Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, Schaffler MB (2005) In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci USA 102(33):11911–11916. doi:10.1073/pnas.0505193102
Wang Y, McNamara LM, Schaffler MB, Weinbaum S (2007) A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci 104(40):15941–15946. doi:10.1073/pnas.0707246104
Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27(3):339–360
Westbroek I, Ajubi NE, Alblas MJ, Semeins CM, Klein-Nulend J, Burger EH, Nijweide PJ (2000) Differential stimulation of prostaglandin G/H synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem Biophys Res Commun 268(2):414–419. doi:10.1006/bbrc.2000.2154
You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR (2000) Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng 122(4):387–393
You L-D, Weinbaum S, Cowin SC, Schaffler MB (2004) Ultrastructure of the osteocyte process and its pericellular matrix. Anatomical Rec A Discov Mol Cell Evol Biol 278A(2):505–513. doi:10.1002/ar.a.20050
You L, Cowin SC, Schaffler MB, Weinbaum S (2001) A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech 34(11):1375–1386
Zeng Y, Cowin S, Weinbaum S (1994) A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann Biomed Eng 22(3):280–292. doi:10.1007/bf02368235
Acknowledgments
The authors would like to acknowledge funding from the Irish Research Council for Science, Engineering and Technology (IRCSET), under the EMBARK program (S. W. V.), the European Research Council (ERC) under grant number 258992 (BONEMECHBIO) and the Irish Centre for High-End Computing (ICHEC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verbruggen, S.W., Vaughan, T.J. & McNamara, L.M. Fluid flow in the osteocyte mechanical environment: a fluid–structure interaction approach. Biomech Model Mechanobiol 13, 85–97 (2014). https://doi.org/10.1007/s10237-013-0487-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-013-0487-y