Abstract
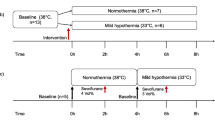
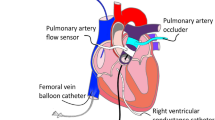
In acute heart failure, systemic arterial pressure (AP), cardiac output (CO), and left atrial pressure (P LA) have to be controlled within acceptable ranges. Under this condition, cardiac energetic efficiency should also be improved. Theoretically, if heart rate (HR) is reduced while AP, CO, and P LA are maintained by preserving the functional slope of left ventricular (LV) Starling’s curve (S L) with precisely increased LV end-systolic elastance (E es), it is possible to improve cardiac energetic efficiency and reduce LV oxygen consumption per minute (MVO 2). We investigated whether this hemodynamics can be accomplished in acute heart failure using an automated hemodynamic regulator that we developed previously. In seven anesthetized dogs with acute heart failure (CO < 70 mL min−1 kg−1, P LA > 15 mmHg), the regulator simultaneously controlled S L with dobutamine, systemic vascular resistance with nitroprusside and stressed blood volume with dextran or furosemide, thereby controlling AP, CO, and P LA. Normal hemodynamics were restored and maintained (CO; 88 ± 3 mL min−1 kg−1, P LA; 10.9 ± 0.4 mmHg), even when zatebradine significantly reduced HR (−27 ± 3%). Following HR reduction, E es increased (+34 ± 14%), LV mechanical efficiency (stroke work/oxygen consumption) increased (+22 ± 6%), and MVO 2 decreased (−17 ± 4%) significantly. In conclusion, in a canine acute heart failure model, computationally managed bradycardia improved cardiac energetic efficiency while restoring normal hemodynamic conditions.





Similar content being viewed by others
References
Antman E. M., D. T. Anbe, P. W. Armstrong, E. R. Bates, L. A. Green, M. Hand, J. S. Hochman, H. M. Krumholz, F. G. Kushner, G. A. Lamas, C. J. Mullany, J. P. Ornato, D. L. Pearle, M. A. Sloan, S. C. Smith Jr., J. S. Alpert, J. L. Anderson, D. P. Faxon, V. Fuster, R. J. Gibbons, G. Gregoratos, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, A. K. Jacobs, J. P. Ornato (2004) American College of Cardiology; American Heart Association; Canadian Cardiovascular Society ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J. Am. Coll. Cardiol. 44, 671–719. doi:10.1016/j.jacc.2004.07.002
Bein B., F. Worthmann, P. H. Tonner, A. Paris, M. Steinfath, J. Hedderich (2004) Comparison of esophageal Doppler, pulse contour analysis, and real-time pulmonary artery thermodilution for the continuous measurement of cardiac output. J. Cardiothorac. Vasc. Anesth. 18, 185–189. doi:10.1053/j.jvca.2004.01.025
Binkley P. F., K. D. Murray, K. M. Watson, P. D. Myerowitz, C. V. Leier (1991) Dobutamine increases cardiac output of the total artificial heart. Implications for vascular contribution of inotropic agents to augmented ventricular function. Circulation 84, 1210–1215
Borer J. S., K. Fox, P. Jaillon, G. Lerebours (2003) Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation 107, 817–823. doi:10.1161/01.CIR.0000048143.25023.87
Choong C. Y., G. S. Roubin, P. J. Harris, Y. Tokuyasu, W. F. Shen, G. J. Bautovich, D. T. Kelly (1986) A comparison of the effects of beta-blockers with and without intrinsic sympathomimetic activity on hemodynamics and left ventricular function at rest and during exercise in patients with coronary artery disease. J. Cardiovasc. Pharmacol. 8, 441–448. doi:10.1097/00005344-198605000-00001
Colin P., B. Ghaleh, X. Monnet, J. Su, L. Hittinger, J. F. Giudicelli, A. Berdeaux (2003) Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am. J. Physiol. Heart Circ. Physiol. 284, H676–H682
Colucci W. S. (1989) Myocardial and vascular actions of milrinone. Eur. Heart J. 10(Suppl C), 32–38
Freeman G. L., W. C. Little, R. A. O’Rourke (1987) Influence of heart rate on left ventricular performance in conscious dogs. Circ. Res. 61, 455–464
Gingrich K. J., R. J. Roy (1991) Modeling the hemodynamic response to dopamine in acute heart failure. IEEE Trans. Biomed. Eng. 38, 267–272. doi:10.1109/10.133208
Glantz S. A. (1976) Ventricular pressure–volume curve indices change with end-diastolic pressure. Circ. Res. 39, 772–778
Glower D. D., J. A. Spratt, N. D. Snow, J. S. Kabas, J. W. Davis, C. O. Olsen, G. S. Tyson, D. C. Sabiston Jr., J. S. Rankin (1985) Linearity of the Frank–Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71, 994–1009
Guth B. D., T. Dietze (1995) If current mediates β-adrenergic enhancement of heart rate but not contractility in vivo. Basic Res. Cardiol. 90, 192–202. doi:10.1007/BF00805662
Hamlin R. L., T. Nakayama, H. Nakayama, C. A. Carnes (2003) Effects of changing heart rate on electrophysiological and hemodynamic function in the dog. Life Sci. 72, 1919–1930. doi:10.1016/S0024-3205(03)00015-8
Hayashi K., K. Shigemi, T. Shishido, M. Sugimachi, K. Sunagawa (2000) Single-beat estimation of ventricular end-systolic elastance-effective arterial elastance as an index of ventricular mechanoenergetic performance. Anesthesiology 92, 1769–1776. doi:10.1097/00000542-200006000-00037
Hayashi Y., M. Takeuchi, H. Takaoka, K. Hata, M. Mori, M. Yokoyama (1996) Alteration in energetics in patients with left ventricular dysfunction after myocardial infarction: increased oxygen cost of contractility. Circulation 93, 932–939
Hettrick D. A., P. S. Pagel, D. Lowe, J. P. Tessmer, D. C. Warltier (1996) Increases in inotropic state without change in heart rate: combined use of dobutamine and zatebradine in conscious dogs. Eur. J. Pharmacol. 316, 237–244. doi:10.1016/S0014-2999(96)00688-7
Hoeksel S. A., J. A. Blom, J. R. Jansen, J. G. Maessen, J. J. Schreuder (1999) Automated infusion of vasoactive and inotropic drugs to control arterial and pulmonary pressures during cardiac surgery. Crit. Care Med. 27, 2792–2798. doi:10.1097/00003246-199912000-00031
Iellamo F., J. A. Sala-Mercado, M. Ichinose, R. L. Hammond, M. Pallante, T. Ichinose, L. W. Stephenson, D. S. O’Leary (2007) Spontaneous baroreflex control of heart rate during exercise and muscle metaboreflex activation in heart failure. Am. J. Physiol. Heart Circ. Physiol. 293, H1929–H1936. doi:10.1152/ajpheart.00564.2007
Kim I. S., H. Izawa, T. Sobue, H. Ishihara, F. Somura, T. Nishizawa, K. Nagata, M. Iwase, M. Yokota (2002) Prognostic value of mechanical efficiency in ambulatory patients with idiopathic dilated cardiomyopathy in sinus rhythm. J. Am. Coll. Cardiol. 39, 1264–1268. doi:10.1016/S0735-1097(02)01775-8
Knaapen P., T. Germans, J. Knuuti, W. J. Paulus, P. A. Dijkmans, C. P. Allaart, A. A. Lammertsma, F. C. Visser (2007) Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation 115, 918–927. doi:10.1161/CIRCULATIONAHA.106.660639
Miyata H., E. G. Lakatta, M. D. Stern, H. S. Silverman (1992) Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ. Res. 71, 605–613
Nikolaidis L. A., T. Hentosz, A. Doverspike, R. Huerbin, C. Stolarski, Y. T. Shen, R. P. Shannon (2002) Catecholamine stimulation is associated with impaired myocardial O2 utilization in heart failure. Cardiovasc. Res. 53, 392–404. doi:10.1016/S0008-6363(01)00490-4
Rao R. R., B. Aufderheide, B. W. Bequette (2003) Experimental studies on multiple-model predictive control for automated regulation of hemodynamic variables. IEEE Trans. Biomed. Eng. 50, 277–288. doi:10.1109/TBME.2003.808813
Schipke J. D., Y. Harasawa, S. Sugiura, J. Alexander Jr., D. Burkhoff (1991) Effect of a bradycardic agent on the isolated blood-perfused canine heart. Cardiovasc. Drugs Ther. 5, 481–488
Shen W., R. M. Gill, B. D. Jones, J. P. Zhang, A. K. Corbly, M. I. Steinberg (2002) Combined inotropic and bradycardic effects of a sodium channel enhancer in conscious dogs with heart failure: a mechanism for improved myocardial efficiency compared with dobutamine. J. Pharmacol. Exp. Ther. 303, 673–680. doi:10.1124/jpet.303.2.673
Shinke T., M. Takeuchi, H. Takaoka, M. Yokoyama (1999) Beneficial effects of heart rate reduction on cardiac mechanics and energetics in patients with left ventricular dysfunction. Jpn. Circ. J. 63, 957–964. doi:10.1253/jcj.63.957
Smiseth O. A., O. D. Mjos (1982) A reproducible and stable model of acute ischaemic left ventricular failure in dogs. Clin. Physiol. 2, 225–239. doi:10.1111/j.1475-097X.1982.tb00027.x
Suga H. (1990) Ventricular energetics. Physiol. Rev. 70, 247–277
Suga H, Y. Yasumura, T. Nozawa, S. Futaki, Y. Igarashi, Y. Goto (1987) Prospective prediction of O2 consumption from pressure–volume area in dog hearts. Am. J. Physiol. 252, H1258–H1264
Uemura K., A. Kamiya, I. Hidaka, T. Kawada, S. Shimizu, T. Shishido, M. Yoshizawa, M. Sugimachi, K. Sunagawa (2006) Automated drug delivery system to control systemic arterial pressure, cardiac output, and left heart filling pressure in acute decompensated heart failure. J. Appl. Physiol. 100, 1278–1286. doi:10.1152/japplphysiol.01206.2005
Uemura K., T. Kawada, A. Kamiya, T. Aiba, I. Hidaka, K. Sunagawa, M. Sugimachi (2005) Prediction of circulatory equilibrium in response to changes in stressed blood volume. Am. J. Physiol. Heart Circ. Physiol. 289, H301–H307. doi:10.1152/ajpheart.01237.2004
Uemura K., M. Sugimachi, T. Kawada, A. Kamiya, Y. Jin, K. Kashihara, K. Sunagawa (2004) A novel framework of circulatory equilibrium. Am. J. Physiol. Heart Circ. Physiol. 286, H2376–H2385. doi:10.1152/ajpheart.00654.2003
Yu C., R. J. Roy, H. Kaufman, B. W. Bequette (1992) Multiple-model adaptive predictive control of mean arterial pressure and cardiac output. IEEE Trans. Biomed. Eng. 39, 765–778. doi:10.1109/10.148385
Acknowledgment
This study was supported by Grant-in-Aid for Scientific Research (C) (18500358, 20500404) from the Ministry of Education, Culture, Sports, Science and Technology, by a research grant from Nakatani Foundation of Electronic Measuring Technology Advancement, and by Health and Labour Sciences Research Grants (H19-nano-ippan-009) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
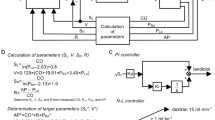
Feedback Control Algorithms of the Automatic Hemodynamic Regulator
To minimize the difference between target and subject’s S L (ΔS L = target S L—subject’s S L) and those of R (ΔR = target R—subject’s R), the proportional-integral (PI) feedback controllers adjust the infusion rates of DOB and SNP, respectively (Fig. 2). In the PI controller (Fig. 6), ΔS L (or ΔR) and the difference integrated with an integral gain (K i) are summed and scaled by a proportional gain (K p) to give the infusion rate of DOB (or SNP). PI gain constants for DOB infusion [K i = 0.01 s−1, K p = 0.06 μg kg−1 min−1 (mL min−1 kg−1)−1] and for SNP infusion [K i = 0.007 s−1, K p = −1.37 μg kg−1 min−1 (mmHg min ml−1 kg)−1] were determined on the basis of open-loop response of S L and R to the infusion of DOB and SNP, respectively.30
To minimize the difference between target and subject’s V (ΔV = target V—subject’s V), a nonlinear (N-L) feedback controller (Fig. 2) adjusts the infusion of DEX or injection of FUR based on the following “if-then” rules:
-
Rule 1: If ΔV ≥ 1 mL kg−1 then infuse DEX at 10 mL min−1
-
Rule 2: If ΔV ≤ −2 mL kg−1 then inject FUR (10 mg) at 10 min intervals
The “if-then” rules were determined on the basis of the open-loop response of V to the infusion of DEX and FUR.30
Rights and permissions
About this article
Cite this article
Uemura, K., Sunagawa, K. & Sugimachi, M. Computationally Managed Bradycardia Improved Cardiac Energetics While Restoring Normal Hemodynamics in Heart Failure. Ann Biomed Eng 37, 82–93 (2009). https://doi.org/10.1007/s10439-008-9595-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9595-8