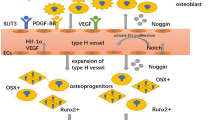
Abstract
Vascularization of large bone grafts is one of the main challenges of bone tissue engineering (BTE), and has held back the clinical translation of engineered bone constructs for two decades so far. The ultimate goal of vascularized BTE constructs is to provide a bone environment rich in functional vascular networks to achieve efficient osseointegration and accelerate restoration of function after implantation. To attain both structural and vascular integration of the grafts, a large number of biomaterials, cells, and biological cues have been evaluated. This review will present biological considerations for bone function restoration, contemporary approaches for clinical salvage of large bone defects and their limitations, state-of-the-art research on the development of vascularized bone constructs, and perspectives on evaluating and implementing novel BTE grafts in clinical practice. Success will depend on achieving full graft integration at multiple hierarchical levels, both between the individual graft components as well as between the implanted constructs and their surrounding host tissues. The paradigm of vascularized tissue constructs could not only revolutionize the progress of BTE, but could also be readily applied to other fields in regenerative medicine for the development of new innovative vascularized tissue designs.





Similar content being viewed by others
References
Aguirre, A., J. A. Planell, and E. Engel. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem. Biophys. Res. Commun. 400:284–291, 2010.
Akita, S., N. Tamai, A. Myoui, M. Nishikawa, T. Kaito, K. Takaoka, and H. Yoshikawa. Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng. 10:789–795, 2004.
Albrektsson, T., and C. Johansson. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 10:S96–S101, 2001.
American Society for Testing and Materials. ASTM F756-00: Standard Practice for Assessment of Hemolytic Properties of Materials. Philadelphia, PA: ASTM International, 2000.
An, Y. H. Mechanical properties of bone. In: Mechanical Testing of Bone and the Bone-Implant Interface, edited by Y. H. An, and R. A. Draughn. Boca Raton, FL: CRC Press, 2000, pp. 41–63.
Annabi, N., S. M. Mithieux, A. S. Weiss, and F. Dehghani. Cross-linked open-pore elastic hydrogels based on tropoelastin, elastin and high pressure CO2. Biomaterials 31:1655–1665, 2010.
Baranski, J. D., R. R. Chaturvedi, K. R. Stevens, J. Eyckmans, B. Carvalho, R. D. Solorzano, M. T. Yang, J. S. Miller, S. N. Bhatia, and C. S. Chen. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA 110:7586–7591, 2013.
Bauer, T., and G. Muschler. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 371:10–27, 2000.
Bergers, G., and S. Song. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 7:452–464, 2005.
Berner, A., J. Boerckel, S. Saifzadeh, R. Steck, J. Ren, C. Vaquette, J. Q. Zhang, M. Nerlich, R. E. Guldberg, and D. Hutmacher. Biomimetic tubular nanofiber mesh and platelet rich plasma-mediated delivery of BMP-7 for large bone defect regeneration. Cell Tissue Res. 347:603–612, 2012.
Betz, R. Limitations of autograft and allograft: new synthetic solutions. Orthopedics 25:s561–s570, 2002.
Bi, Y., C. H. Stuelten, T. Kilts, S. Wadhwa, R. V. Iozzo, P. G. Robey, X.-D. Chen, and M. F. Young. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J. Biol. Chem. 280:30481–30489, 2005.
Black, L., P. Purnell, and J. Hill. Current themes in cement research. Adv. Appl. Ceram. 109:253–259, 2010.
Blum, A. L. L., J. C. Bongiovanni, S. J. Morgan, M. A. Flierl, and F. B. dos Reis. Complications associated with distraction osteogenesis for infected nonunion of the femoral shaft in the presence of a bone defect: a retrospective series. J. Bone Joint Surg. Br. 92-B:565–570, 2010.
Bonzani, I. C., R. Adhikari, S. Houshyar, R. Mayadunne, P. Gunatillake, and M. M. Stevens. Synthesis of two-component injectable polyurethanes for bone tissue engineering. Biomaterials 28:423–433, 2007.
Buckwalter, J., M. Glimcher, R. Cooper, and R. Recker. Bone biology. J. Bone Joint Surg. Am. 77:1256–1275, 1995.
Chim, H., C. J. Salgado, S. Mardini, and H.-C. Chen. Reconstruction of mandibular defects. Semin. Plast. Surg. 24:188–197, 2010.
Dabrowski, B., W. Swieszkowski, D. Godlinski, and K. J. Kurzydlowski. Highly porous titanium scaffolds for orthopaedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 95B:53–61, 2010.
Davis, G. E., and D. R. Senger. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97:1093–1107, 2005.
Deb, S., R. Mandegaran, and L. Di Silvio. A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J. Mater. Sci. Mater. Med. 21:893–905, 2010.
Dehghani, F., and N. Annabi. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 22:661–666, 2011.
Drury, J. L., and D. J. Mooney. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24:4337–4351, 2003.
Farshid, B., G. Lalwani, and B. Sitharaman. Cytotoxicity of polypropylene fumarate nanocomposites used in bone tissue engineering. In: 39th Annual Northeast Bioengineering Conference (NEBEC). Syracuse, NY, 2013, pp. 119–120.
Ferreira, C. L., F. A. M. D. Abreu, G. A. B. Silva, F. F. Silveira, L. B. A. Barreto, T. D. P. Paulino, M. N. Miziara, and J. B. Alves. TGF-β1 and BMP-4 carried by liposomes enhance the healing process in alveolar bone. Arch. Oral Biol. 58:646–656, 2013.
Folkman, J., and M. Hochberg. Self-regulation of growth in three dimensions. J. Exp. Med. 138:745–753, 1973.
Glowacki, J., and S. Mizuno. Collagen scaffolds for tissue engineering. Biopolymers 89:338–344, 2008.
Grayson, W. L., M. Fröhlich, K. Yeager, S. Bhumiratana, M. E. Chan, C. Cannizzaro, L. Q. Wan, X. S. Liu, X. E. Guo, and G. Vunjak-Novakovic. Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. USA 107:3299–3304, 2010.
Greenwald, S. E., and C. L. Berry. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J. Pathol. 190:292–299, 2000.
Gurtner, G. C., K. A. Bhatt, and V. W. Wong. Composite tissue engineering and organ regeneration using explanted microvascular beds (EMBs). Plast. Reconstr. Surg. 124:106–107, 2009.
Habibovic, P., and K. de Groot. Osteoinductive biomaterials-properties and relevance in bone repair. J. Tissue Eng. Regen. Med. 1:25–32, 2007.
Haholu, A., C. Sever, F. Uygur, G. Kose, M. Urhan, O. Sinan, O. Omer, S. Cihan, and Y. Kulahci. Prefabrication of vascularized bone graft using an interconnected porous calcium hydroxyapatite ceramic in presence of vascular endothelial growth factor and bone marrow mesenchymal stem cells: experimental study in rats. Indian J. Plast. Surg. 45:444–452, 2012.
Hastings, C. L., H. M. Kelly, M. J. Murphy, F. P. Barry, F. J. O’Brien, and G. P. Duffy. Development of a thermoresponsive chitosan gel combined with human mesenchymal stem cells and desferrioxamine as a multimodal pro-angiogenic therapeutic for the treatment of critical limb ischaemia. J. Control. Release 161:73–80, 2012.
Heslop, B. F., I. M. Zeiss, and N. W. Nisbet. Studies on transference of bone: I. A comparison of autologous and homologous bone implants with reference to osteocyte survival, osteogenesis and host reaction. Br. J. Exp. Pathol. 41:269–287, 1960.
Hess, J. R., R. L. Sparrow, P. F. Van Der Meer, J. P. Acker, R. A. Cardigan, and D. V. Devine. Blood components: red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion 49:2599–2603, 2009.
Ilizarov, G. A. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin. Orthop. Relat. Res. 239:263–285, 1989.
Jain, R. K., P. Au, J. Tam, D. G. Duda, and D. Fukumura. Engineering vascularized tissue. Nat. Biotechnol. 23:821–823, 2005.
Jin, G., and G. Kim. The effect of sinusoidal AC electric stimulation of 3D PCL/CNT and PCL/β-TCP based bio-composites on cellular activities for bone tissue regeneration. J. Mater. Chem. B 1:1439–1452, 2013.
Jung, S., and J. Kleinheinz. Angiogenesis — the key to regeneration. In: Regenerative Medicine and Tissue Engineering, edited by J. A. Andrades. Rijeka: InTech, 2013, pp. 453–473.
Kang, Y., E. Jabbari, and Y. Yang. Integrating top-down and bottom-up scaffolding tissue engineering approach for bone regeneration. In: Micro and Nanotechnologies in Engineering Stem Cells and Tissues, edited by M. Ramalingam, E. Jabbari, S. Ramakrishna, and A. Khademhosseini. Hoboken, NJ: Wiley, 2013, pp. 142–159.
Kang, Y., S. Kim, A. Khademhosseini, and Y. Yang. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials 32:6119–6130, 2011.
Kang, S.-W., J.-S. Kim, K.-S. Park, B.-H. Cha, J.-H. Shim, J. Y. Kim, D.-W. Cho, J.-W. Rhie, and S.-H. Lee. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone 48:298–306, 2011.
Kang, J. K., M. H. Lee, B. J. Kwon, H. H. Kim, I. K. Shim, M. R. Jung, S. J. Lee, and J.-C. Park. Effective layer by layer cell seeding into non-woven 3D electrospun scaffolds of poly-L-lactic acid microfibers for uniform tissue formation. Macromol. Res. 20:795–799, 2012.
Kang, Y., N. Mochizuki, A. Khademhosseini, J. Fukuda, and Y. Yang. Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach. Acta Biomater. 11:449–458, 2015.
Kang, Y., L. Ren, and Y. Yang. Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. ACS Appl. Mater. Interfaces 6:9622–9633, 2014.
Kang, Y., A. Scully, D. A. Young, S. Kim, H. Tsao, M. Sen, and Y. Yang. Enhanced mechanical performance and biological evaluation of a PLGA coated ε-TCP composite scaffold for load-bearing applications. Eur. Polym. J. 47:1569–1577, 2011.
Karger, C., T. Kishi, L. Schneider, F. Fitoussi, and A. C. Masquelet. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 98:97–102, 2012.
Kaully, T., K. Kaufman-Francis, A. Lesman, and S. Levenberg. Vascularization—the conduit to viable engineered tissues. Tissue Eng. Part B Rev. 15:159–169, 2009.
Khira, Y. M., and H. A. Badawy. Pedicled vascularized fibular graft with Ilizarov external fixator for reconstructing a large bone defect of the tibia after tumor resection. J. Orthop. Traumatol. 14:91–100, 2013.
Kim, S., K. Bedigrew, T. Guda, W. J. Maloney, S. Park, J. C. Wenke, and Y. P. Yang. Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects. Acta Biomater. 10:5021–5033, 2014.
Kim, S., Y. Kang, C. A. Krueger, M. Sen, J. B. Holcomb, D. Chen, J. C. Wenke, and Y. Yang. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with gelatin microspheres enhances early osteoblastic differentiation. Acta Biomater. 8:1768–1777, 2012.
Kim, S., Y. Kang, Á. E. Mercado-Pagán, W. J. Maloney, and Y. Yang. In vitro evaluation of photo-crosslinkable chitosan-lactide hydrogels for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 102:1393–1406, 2014.
Kim, S., and Hv. Recum. Endothelial stem cells and precursors for tissue engineering: cell source, differentiation, selection, and application. Tissue Eng. Part B Rev. 14:133–147, 2008.
Kirkpatrick, J. S., C. N. Cornell, B. H. Hoang, W. Hsu, J. T. Watson, W. C. Watters, C. M. Turkelson, J. L. Wies, and S. Anderson. Bone void fillers. J. Am. Acad. Orthop. Surg. 18:576–579, 2010.
Kneser, U., E. Polykandriotis, J. Ohnolz, K. Heidner, L. Grabinger, S. Euler, K. U. Amann, A. Hess, K. Brune, and P. Greil. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 12:1721–1731, 2006.
Kneser, U., D. Schaefer, E. Polykandriotis, and R. Horch. Tissue engineering of bone: the reconstructive surgeon’s point of view. J. Cell Mol. Med. 10:7–19, 2006.
Koffler, J., K. Kaufman-Francis, Y. Shandalov, D. Egozi, D. Amiad Pavlov, A. Landesberg, and S. Levenberg. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc. Natl. Acad. Sci. USA 108:14789–14794, 2011.
Kramschuster, A., and L.-S. Turng. An injection molding process for manufacturing highly porous and interconnected biodegradable polymer matrices for use as tissue engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 92B:366–376, 2010.
Krishnan, L., N. Willett, and R. Guldberg. Vascularization strategies for bone regeneration. Ann. Biomed. Eng. 42:432–444, 2014.
Langer, R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng. 13:1–2, 2007.
Laschke, M. W., Y. Harder, M. Amon, I. Martin, J. Farhadi, A. Ring, N. Torio-Padron, R. Schramm, M. Rücker, and D. Junker. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 12:2093–2104, 2006.
Laschke, M. W., H. Mussawy, S. Schuler, A. Kazakov, M. Rücker, D. Eglin, M. Alini, and M. D. Menger. Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue. Tissue Eng. Part A 17:841–853, 2010.
Lawson, J., S. Dahl, H. Prichard, R. Manson, S. Gage, A. Kypson, J. Blum, A. Pilgrim, W. Tente, and L. Niklason. VS5 human tissue-engineered grafts for hemodialysis: development, preclinical data, and early investigational human implant experience. J. Vasc. Surg. 59:32S–33S, 2014.
Lee, J.-H., J.-H. Kim, S.-H. Oh, S.-J. Kim, Y.-S. Hah, B.-W. Park, D. R. Kim, G.-J. Rho, G.-H. Maeng, R.-H. Jeon, H.-C. Lee, J.-R. Kim, G.-C. Kim, U.-K. Kim, and J.-H. Byun. Tissue-engineered bone formation using periosteal-derived cells and polydioxanone/pluronic F127 scaffold with pre-seeded adipose tissue-derived CD146 positive endothelial-like cells. Biomaterials 32:5033–5045, 2011.
L’Heureux, N., S. Pâquet, R. Labbé, L. Germain, and F. A. Auger. A completely biological tissue-engineered human blood vessel. FASEB J. 12:47–56, 1998.
Liao, J., L. Zhang, Y. Zuo, H. Wang, J. Li, Q. Zou, and Y. Li. Development of nanohydroxyapatite/polycarbonate composite for bone repair. J. Biomater. Appl. 24:31–45, 2009.
Liberti, L., A. Breckenridge, H. G. Eichler, R. Peterson, N. McAuslane, and S. Walker. Expediting patients’ access to medicines by improving the predictability of drug development and the regulatory approval process. Clin. Pharmacol. Ther. 87:27–31, 2009.
Liska, R., M. Schuster, R. Inführ, C. Turecek, C. Fritscher, B. Seidl, V. Schmidt, L. Kuna, A. Haase, F. Varga, H. Lichtenegger, and J. Stampfl. Photopolymers for rapid prototyping. J. Coat. Techol. Res. 4:505–510, 2007.
Liu, G., W. Fan, X. Miao, Y. Xiao, D. Good, and M. Q. Wei. Sequential release of BMP-7 and VEGF from the PLGA/AK-gelatin composite scaffolds. J. Biomim. Biomater. Tissue Eng. 11:81–91, 2011.
Liu, Y., J. H. Kim, D. Young, S. K. Nishimoto, R. Heck, and Y. Yang. Biomimetic macroporous scaffolds with high mechanical strength and biological evaluation. In: 38th Annual Meeting of the American Association for Dental Research. Miami, FL, 2009, p. 120808.
Liu, Y., S.-H. Teoh, M. S. K. Chong, C.-H. Yeow, R. D. Kamm, M. Choolani, and J. K. Y. Chan. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng. Part A 19:893–904, 2012.
Macdonald, M. L., R. E. Samuel, N. J. Shah, R. F. Padera, Y. M. Beben, and P. T. Hammond. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 32:1446–1453, 2011.
Marolt, D., I. M. Campos, S. Bhumiratana, A. Koren, P. Petridis, G. Zhang, P. F. Spitalnik, W. L. Grayson, and G. Vunjak-Novakovic. Engineering bone tissue from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 109:8705–8709, 2012.
Masquelet, A. C., and T. Begue. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 41:27–37, 2010.
Masquelet, A., F. Fitoussi, T. Begue, and G. Muller. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 45:346–353, 2000.
Matsumoto, T., A. Kawamoto, R. Kuroda, M. Ishikawa, Y. Mifune, H. Iwasaki, M. Miwa, M. Horii, S. Hayashi, A. Oyamada, H. Nishimura, S. Murasawa, M. Doita, M. Kurosaka, and T. Asahara. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am. J. Pathol. 169:1440–1457, 2006.
Mauffrey, C., M. Madsen, R. J. Bowles, and D. Seligson. Bone graft harvest site options in orthopaedic trauma: a prospective in vivo quantification study. Injury 43:323–326, 2012.
McCarthy, I. The physiology of bone blood flow: a review. J. Bone Joint Surg. Am. 88:4–9, 2006.
McFadden, T. M., G. P. Duffy, A. B. Allen, H. Y. Stevens, S. M. Schwarzmaier, N. Plesnila, J. M. Murphy, F. P. Barry, R. E. Guldberg, and F. J. O’Brien. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen–glycosaminoglycan scaffold in vivo. Acta Biomater. 9:9303–9316, 2013.
Meng, Z. X., H. F. Li, Z. Z. Sun, W. Zheng, and Y. F. Zheng. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Mater. Sci. Eng. C 33:699–706, 2013.
Mercado, Á. E., and Y. Yang. Strategies towards engineering vascularized bone graft substitutes. In: Bone Graft Substitutes and Bone Regenerative Engineering, edited by C. Laurencin, and T. Jiang. West Conshohocken, PA: ASTM International, 2014, pp. 299–334.
Mercado, A. E., X. Yang, X. He, and E. Jabbari. Effect of grafting BMP2-derived peptide to nanoparticles on osteogenic and vasculogenic expression of stromal cells. J. Tissue Eng. Regen. Med. 8:15–28, 2014.
Mercado-Pagán, Á. E., Y. Kang, D. F. E. Ker, S. Park, J. Yao, J. Bishop, and Y. P. Yang. Synthesis and characterization of novel elastomeric poly(D, L-lactide urethane) maleate composites for bone tissue engineering. Eur. Polym. J. 49:3337–3349, 2013.
Mercado-Pagán, Á. E., D. F. E. Ker, and Y. Yang. Hemocompatibility evaluation of small elastomeric hollow fiber membranes as vascular substitutes. J. Biomater. Appl. 29:557–565, 2014.
Miller, J. S., K. R. Stevens, M. T. Yang, B. M. Baker, D.-H. T. Nguyen, D. M. Cohen, E. Toro, A. A. Chen, P. A. Galie, X. Yu, R. Chaturvedi, S. N. Bhatia, and C. S. Chen. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11:768–774, 2012.
Mizrahi, O., D. Sheyn, W. Tawackoli, I. Kallai, A. Oh, S. Su, X. Da, P. Zarrini, G. Cook-Wiens, D. Gazit, and Z. Gazit. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 20:370–377, 2013.
Morozowich, N. L., J. L. Nichol, and H. R. Allcock. Investigation of apatite mineralization on antioxidant polyphosphazenes for bone tissue engineering. Chem. Mater. 24:3500–3509, 2012.
Moshaverinia, A., S. Ansari, C. Chen, X. Xu, K. Akiyama, M. L. Snead, H. H. Zadeh, and S. Shi. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials 34:6572–6579, 2013.
Nauth, A., P. V. Giannoudis, T. A. Einhorn, K. D. Hankenson, G. E. Friedlaender, R. Li, and E. H. Schemitsch. Growth factors: beyond bone morphogenetic proteins. J. Orthop. Trauma 24:543–546, 2010.
Neff, L. P., B. W. Tillman, S. K. Yazdani, M. A. Machingal, J. J. Yoo, S. Soker, B. W. Bernish, R. L. Geary, and G. J. Christ. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J. Vasc. Surg. 53:426–434, 2011.
Nguyen, L. H., N. Annabi, M. Nikkhah, H. Bae, L. Binan, S. Park, Y. Kang, Y. Yang, and A. Khademhosseini. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng. Part B Rev. 18:363–382, 2012.
Nguyen, B.-N. B., and J. P. Fisher. In vivo techniques and strategies for enhanced vascularization of engineered bone. In: Vascularization—Regenerative Medicine and Tissue Engineering, edited by E. M. Brey. Boca Raton, FL: CRC Press, 2014, pp. 263–282.
Papadimitropoulos, A., A. Scherberich, S. Güven, N. Theilgaard, H. J. A. Crooijmans, F. Santini, K. Scheffler, A. Zallone, and I. Martin. A 3D in vitro bone organ model using human progenitor cells. Eur. Cells Mater. 21:445–458, 2011.
Papakostidis, C., M. Bhandari, and P. Giannoudis. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Joint J. 95:1673–1680, 2013.
Patel, Z. S., S. Young, Y. Tabata, J. A. Jansen, M. E. K. Wong, and A. G. Mikos. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43:931–940, 2008.
Patterson, J., R. Siew, S. W. Herring, A. S. Lin, R. Guldberg, and P. S. Stayton. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 31:6772–6781, 2010.
Pepper, M. S. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 8:21–43, 1997.
Phemister, D. B. Changes in bones and joints resulting from interruption of circulation: I. General considerations and changes resulting from injuries. Arch. Surg. 41:436–472, 1940.
Pondrom, S. The AJT report: news and issues that affect organ and tissue transplantation. Am. J. Transplant. 10:1953–1954, 2010.
Poshusta, A. K., J. A. Burdick, D. J. Mortisen, R. F. Padera, D. Ruehlman, M. J. Yaszemski, and K. S. Anseth. Histocompatibility of photocrosslinked polyanhydrides: a novel in situ forming orthopaedic biomaterial. J. Biomed. Mater. Res. A 64:62–69, 2003.
Prodanov, L., C. M. Semeins, J. J. W. A. van Loon, J. te Riet, J. A. Jansen, J. Klein-Nulend, and X. F. Walboomers. Influence of nanostructural environment and fluid flow on osteoblast-like cell behavior: a model for cell-mechanics studies. Acta Biomater. 9:6653–6662, 2013.
Qu, D., J. Li, Y. Li, Y. Gao, Y. Zuo, Y. Hsu, and J. Hu. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J. Biomed. Mater. Res. A 96A:543–551, 2011.
Ritchie, R. O. The conflicts between strength and toughness. Nat. Mater. 10:817–822, 2011.
Rivron, N., J. Liu, J. Rouwkema, J. de Boer, and C. van Blitterswijk. Engineering vascularised tissues in vitro. Eur. Cells Mater. 15:27–40, 2008.
Rouwkema, J., P. E. Westerweel, J. de Boer, M. C. Verhaar, and C. A. van Blitterswijk. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng. Part A 15:2015–2027, 2009.
Santos, M. I., K. Tuzlakoglu, S. Fuchs, M. E. Gomes, K. Peters, R. E. Unger, E. Piskin, R. L. Reis, and C. J. Kirkpatrick. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials 29:4306–4313, 2008.
Sen, M., and T. Miclau. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 38:S75–S80, 2007.
Shanjani, Y., Y. Hu, E. Toyserkani, M. Grynpas, R. A. Kandel, and R. M. Pilliar. Solid freeform fabrication of porous calcium polyphosphate structures for bone substitute applications: in vivo studies. J. Biomed. Mater. Res. B Appl. Biomater. 101B:972–980, 2013.
Sheng, M. H. C., K. H. W. Lau, and D. J. Baylink. Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J. Bone Metab. 21:41–54, 2014.
Staiger, M. P., A. M. Pietak, J. Huadmai, and G. Dias. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 27:1728–1734, 2006.
Stevens, M. M. Biomaterials for bone tissue engineering. Mater. Today 11:18–25, 2008.
Sun, Y., C. Zhang, D. Jin, J. Sheng, X. Cheng, X. Liu, S. Chen, and B. Zeng. Free vascularised fibular grafting in the treatment of large skeletal defects due to osteomyelitis. Int. Orthop. 34:425–430, 2010.
Taylor, B. C., B. G. French, T. T. Fowler, J. Russell, and A. Poka. Induced membrane technique for reconstruction to manage bone loss. J. Am. Acad. Orthop. Surg. 20:142–150, 2012.
Teixeira, S., H. Fernandes, A. Leusink, C. van Blitterswijk, M. P. Ferraz, F. J. Monteiro, and J. de Boer. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 93A:567–575, 2010.
Therriault, D., S. R. White, and J. A. Lewis. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2:265–271, 2003.
Tremblay, P.-L., V. Hudon, F. Berthod, L. Germain, and F. A. Auger. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 5:1002–1010, 2005.
Unger, R. E., K. Peters, M. Wolf, A. Motta, C. Migliaresi, and C. J. Kirkpatrick. Endothelialization of a non-woven silk fibroin net for use in tissue engineering: growth and gene regulation of human endothelial cells. Biomaterials 25:5137–5146, 2004.
Wu, W., R. A. Allen, and Y. Wang. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 18:1148–1153, 2012.
Yang, Y., Y. Kang, M. Sen, and S. Park. Bioceramics in tissue engineering. In: Biomaterials for Tissue Engineering: A Review of the Past and Future Trends, edited by J. Burdick, and R. Mauck. New York, NY: Springer Wien, 2010, pp. 179–208.
Yang, H., J. Li, Z. Zhou, and J. Ruan. Structural preparation and biocompatibility evaluation of highly porous tantalum scaffolds. Mater. Lett. 100:152–155, 2013.
Yang, P., C. Wang, Z. Shi, X. Huang, X. Dang, S. Xu, and K. Wang. Prefabrication of vascularized porous three-dimensional scaffold induced from rhVEGF165: a preliminary study in rats. Cells Tissues Organs 189:327–337, 2009.
Zanetti, A. S., C. Sabliov, J. M. Gimble, and D. J. Hayes. Human adipose-derived stem cells and three-dimensional scaffold constructs: A review of the biomaterials and models currently used for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 101B:187–199, 2013.
Zeng, X., Y.-S. Zeng, Y.-H. Ma, L.-Y. Lu, B.-L. Du, W. Zhang, Y. Li, and W. Y. Chan. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant. 20:1881–1899, 2011.
Zheng, Y., J. Chen, M. Craven, N. W. Choi, S. Totorica, A. Diaz-Santana, P. Kermani, B. Hempstead, C. Fischbach-Teschl, J. A. López, and A. D. Stroock. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 109:9342–9347, 2012.
Zimmermann, G., and A. Moghaddam. Allograft bone matrix versus synthetic bone graft substitutes. Injury 42(Supplement 2):S16–S21, 2011.
Zioupos, P., and J. D. Currey. The extent of microcracking and the morphology of microcracks in damaged bone. J. Mater. Sci. 29:978–986, 1994.
Acknowledgments
We would like to acknowledge the financial support of the following agencies: NIH R01AR057837 (NIAMS), NIH R01DE021468 (NIDCR), and DOD W81XWH-10-1-0966 (PRORP).
Conflict of interest
The authors have no conflicts of interest with respect to this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Nadya Lumelsky oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Mercado-Pagán, Á.E., Stahl, A.M., Shanjani, Y. et al. Vascularization in Bone Tissue Engineering Constructs. Ann Biomed Eng 43, 718–729 (2015). https://doi.org/10.1007/s10439-015-1253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1253-3