Abstract
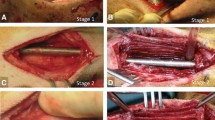
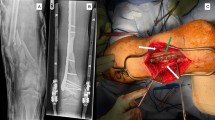
We and others have shown that changing surface characteristics of the spacer implanted during the first Masquelet stage alters some aspects of membrane development. Previously we demonstrated that titanium (TI) spacers create membranes that are better barriers to movement of solutes > 70 kDa in size than polymethyl methacrylate (PMMA) induced-membranes, and roughening creates more mechanically compliant membranes. However, it is unclear if these alterations affect the membrane’s biochemical environment or bone regeneration during the second stage. Ten-week-old, male Sprague–Dawley rats underwent an initial surgery to create an externally stabilized 6 mm femoral defect. PMMA or TI spacers with smooth (~ 1 μm) or roughened (~ 8 μm) surfaces were implanted. Four weeks later, rats were either euthanized for membrane harvest or underwent the second Masquelet surgery. TI spacers induced thicker membranes that were similar in structure and biochemical expression. All membranes were bilayered with the inner layer having increased factor expression [bone morphogenetic protein 2 (BMP2), transforming growth factor beta (TGFβ), interleukin 6 (IL6), and vascular endothelial growth factor (VEGF)]. Roughening increased overall IL6 levels. Ten-weeks post-engraftment, PMMA-smooth induced membranes better supported bone regeneration (60% union). The other groups only had 1 or 2 that united (9–22%). There were no significant differences in any micro computed tomography or dynamic histology outcome. In conclusion, this study suggests that the membrane’s important function in the Masquelet technique is not simply as a barrier. There is likely a critical biochemical, cellular, or vascular component as well.












Similar content being viewed by others
Abbreviations
- PMMA:
-
Polymethyl methacrylate—traditional spacer material, also known as bone cement
- TI:
-
Titanium—experimental spacer material
- PBS:
-
Phosphate buffered saline—wash solution
- DAPI:
-
4′,6-Diamidino-2-phenylindole—nuclear stain
- TGFβ:
-
Transforming growth factor beta—positive regenerative protein
- BMP2:
-
Bone morphogenetic protein 2—positive regenerative protein—promotes osteogenic differentiation
- VEGF:
-
Vascular endothelial growth factor—positive regenerative protein—promotes angiogenesis
- IL6:
-
Interleukin 6—negative regenerative protein—proinflammatory factor
- microCT:
-
Micro computed tomography
- BV/TV :
-
Bone volume/total volume fraction—fraction of volume of interest filled with bone
- TV :
-
Total volume—total volume of interest
- BV :
-
Bone volume—bone within the total volume of interest
- BMD :
-
Bone mineral density—average mineral density of both bone and space within the volume of interest
- TMD :
-
Tissue mineral density—average mineral density of only bone within the volume of interest
References
Ai-Aql, Z. S., A. S. Alagl, D. T. Graves, L. C. Gerstenfeld, and T. A. Einhorn. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 87:107–118, 2008.
Anderson, J. M., A. Rodriguez, and D. T. Chang. Foreign body reaction to biomaterials. Semin. Immunol. 20:86–100, 2008.
Aurégan, J.-C., and T. Bégué. Induced membrane for treatment of critical sized bone defect: a review of experimental and clinical experiences. Int. Orthop. 38:1971–1978, 2014.
Bastian, O., J. Pillay, J. Alblas, L. Leenen, L. Koenderman, and T. Blokhuis. Systemic inflammation and fracture healing. J. Leukoc. Biol. 89:669–673, 2011.
Boskey, A. L., and R. Coleman. Aging and bone. J. Dent. Res. 89:1333–1348, 2010.
Bragdon, B., K. Lybrand, and L. Gerstenfeld. Overview of biological mechanisms and applications of three murine models of bone repair: closed fracture with intramedullary fixation, distraction osteogenesis, and marrow ablation by reaming. Curr. Protoc. Mouse Biol. 5:21–34, 2015.
Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 28–42, 1983. http://www.ncbi.nlm.nih.gov/pubmed/6339139.
Chadayammuri, V., M. Hake, and C. Mauffrey. Innovative strategies for the management of long bone infection: a review of the Masquelet technique. Patient Saf. Surg. 9:32, 2015.
Claes, L., S. Recknagel, and A. Ignatius. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8:133–143, 2012.
Cypher, T. J., and J. P. Grossman. Biological principles of bone graft healing. J. Foot Ankle Surg. 35:413–417, 1996.
Deskins, D. L., S. Ardestani, and P. P. Young. The polyvinyl alcohol sponge model implantation. J. Vis. Exp. 2012. https://doi.org/10.3791/3885.
Dimitriou, R., G. I. Mataliotakis, G. Calori, and P. V. Giannoudis. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med. 10:81, 2012.
Flurkey, K., J. M. Currer, and D. E. Harrison. Chapter 20—mouse models in aging research. In: The Mouse in Biomedical Research, edited by J. G. Fox, M. T. Davisson, F. W. Quimby, S. W. Barthold, C. E. Newcomer, and A. L. Smith. Burlington, MA: Academic, 2007, pp. 637–672. https://doi.org/10.1016/b978-012369454-6/50074-1.
Gaio, N., A. Martino, Z. Toth, J. T. Watson, D. Nicolaou, and S. McBride-Gagyi. Masquelet technique: the effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J. Biomech. 72:53–62, 2018.
Geetha, M., A. K. Singh, R. Asokamani, and A. K. Gogia. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Prog. Mater. Sci. 54:397–425, 2009.
Gerstenfeld, L. C., D. M. Cullinane, G. L. Barnes, D. T. Graves, and T. A. Einhorn. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 88:873–884, 2003.
Giannoudis, P. V., O. Faour, T. Goff, N. Kanakaris, and R. Dimitriou. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury 42:591–598, 2011.
Gibon, E., L. Lu, and S. B. Goodman. Aging, inflammation, stem cells, and bone healing. Stem Cell Res. Ther. 7:44, 2016.
Goriainov, V., R. Cook, J. M. Latham, D. G. Dunlop, and R. O. C. Oreffo. Bone and metal: an orthopaedic perspective on osseointegration of metals. Acta Biomater. 10:4043–4057, 2014.
Gouron, R., L. Petit, C. Boudot, I. Six, M. Brazier, S. Kamel, and R. Mentaverri. Osteoclasts and their precursors are present in the induced-membrane during bone reconstruction using the Masquelet technique. J. Tissue Eng. Regen. Med. 11:382–389, 2014.
Gruber, H. E., F. K. Gettys, H. E. Montijo, J. S. Starman, E. Bayoumi, K. J. Nelson, G. L. Hoelscher, W. K. Ramp, N. Zinchenko, J. A. Ingram, M. J. Bosse, and J. F. Kellam. Genomewide molecular and biologic characterization of biomembrane formation adjacent to a methacrylate spacer in the rat femoral segmental defect model. J. Orthop. Trauma 27:290–297, 2013.
Gruber, H. E., G. Ode, G. Hoelscher, J. Ingram, S. Bethea, and M. J. Bosse. Osteogenic, stem cell and molecular characterisation of the human induced membrane from extremity bone defects. Bone Jt Res. 5:106–115, 2016.
Hadjiargyrou, M., and R. J. O’Keefe. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J. Bone Miner. Res. 29:2307–2322, 2014.
Hotchen, A. J., L. V. Barr, and M. Krkovic. Bridging hard callus at 48 days in an open femoral shaft fracture with segmental defect treated with a first-stage Masquelet technique: I wasn’t expecting that. Strateg. Trauma Limb Reconstr. 13:57–60, 2018.
Karger, C., T. Kishi, L. Schneider, F. Fitoussi, and A.-C. Masquelet. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 98:97–102, 2012.
Kelly, D. J., and C. R. Jacobs. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res. C 90:75–85, 2010.
Kenneth Ward, W. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2:768–777, 2008.
Khan, S. N., F. P. Cammisa, H. S. Sandhu, A. D. Diwan, F. P. Girardi, and J. M. Lane. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 13:77–86, 2005.
Klaue, K., U. Knothe, C. Anton, D. H. Pfluger, M. Stoddart, A. C. Masquelet, and S. M. Perren. Bone regeneration in long-bone defects: tissue compartmentalisation? In vivo study on bone defects in sheep. Injury 40(Suppl 4):S95–S102, 2009.
Klein-Nulend, J., R. G. Bacabac, and M. G. Mullender. Mechanobiology of bone tissue. Pathol. Biol. (Paris) 53:576–580, 2005.
Kon, T., T.-J. Cho, T. Aizawa, M. Yamazaki, N. Nooh, D. Graves, L. C. Gerstenfeld, and T. A. Einhorn. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 16:1004–1014, 2001.
Liu, H., G. Hu, P. Shang, Y. Shen, P. Nie, L. Peng, and H. Xu. Histological characteristics of induced membranes in subcutaneous, intramuscular sites and bone defect. Orthop. Traumatol. Surg. Res. 99:959–964, 2013.
Loi, F., L. A. Córdova, J. Pajarinen, T. Lin, Z. Yao, and S. B. Goodman. Inflammation, fracture and bone repair. Bone 86:119–130, 2016.
Luangphakdy, V., G. Elizabeth Pluhar, N. S. Piuzzi, J.-C. D’Alleyrand, C. S. Carlson, J. E. Bechtold, J. Forsberg, and G. F. Muschler. The effect of surgical technique and spacer texture on bone regeneration: a caprine study using the Masquelet technique. Clin. Orthop. Relat. Res. 475:2575–2585, 2017.
Macaulay, W., C. W. DiGiovanni, A. Restrepo, K. J. Saleh, H. Walsh, L. S. Crossett, M. G. E. Peterson, S. Li, and E. A. Salvati. Differences in bone-cement porosity by vacuum mixing, centrifugation, and hand mixing. J. Arthroplast. 17:569–575, 2002.
Masquelet, A. C., and T. Begue. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 41:27–37; table of contents, 2010.
Mauffrey, C., B. T. Barlow, and W. Smith. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 23:143–153, 2015.
McBride-Gagyi, S. H., J. A. McKenzie, E. G. Buettmann, M. J. Gardner, and M. J. Silva. Bmp2 conditional knockout in osteoblasts and endothelial cells does not impair bone formation after injury or mechanical loading in adult mice. Bone 81:533–543, 2015.
McBride-Gagyi, S., Z. Toth, D. Kim, V. Ip, E. Evans, J. T. Watson, and D. Nicolaou. Altering spacer material affects bone regeneration in the Masquelet technique in a rat femoral defect. J. Orthop. Res. 2018. https://doi.org/10.1002/jor.23866.
Morelli, I., L. Drago, D. A. George, E. Gallazzi, S. Scarponi, and C. L. Romanò. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury 47:S68–S76, 2016.
Morelli, I., L. Drago, D. A. George, D. Romanò, and C. L. Romanò. Managing large bone defects in children. J. Pediatr. Orthop. B 2017. https://doi.org/10.1097/bpb.0000000000000456.
Morgan, E. F., R. E. Gleason, L. N. M. Hayward, P. L. Leong, and K. T. S. Palomares. Mechanotransduction and fracture repair. J. Bone Jt Surg. Am. 90(Suppl 1):25–30, 2008.
Nau, C., C. Seebach, A. Trumm, A. Schaible, K. Kontradowitz, S. Meier, H. Buechner, I. Marzi, and D. Henrich. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 47:325–334, 2016.
Nuss, K. M. R., and B. von Rechenberg. Biocompatibility issues with modern implants in bone—a review for clinical orthopedics. Open Orthop. J. 2:66–78, 2008.
Prystaz, K., K. Kaiser, A. Kovtun, M. Haffner-Luntzer, V. Fischer, A. E. Rapp, A. Liedert, G. Strauss, G. H. Waetzig, S. Rose-John, and A. Ignatius. Distinct effects of IL-6 classic and trans-signaling in bone fracture healing. Am. J. Pathol. 188:474–490, 2018.
Richards, R. G. Implant surfaces: do they have any relevance to the surgeon? AO Dialogue 07:20–24, 2007.
Roddy, E., M. R. Debaun, A. Daoud-gray, Y. P. Yang, and M. J. Gardner. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2017. https://doi.org/10.1007/s00590-017-2063-0.
Rolfe, B., J. Mooney, B. Zhang, S. Jahnke, S.-J. Le, Y.-Q. Chau, Q. Huang, H. Wang, G. Campbell, and J. Campbell. The fibrotic response to implanted biomaterials: implications for tissue engineering. In: Regenerative Medicine and Tissue Engineering—Cells and Biomaterials. InTech, 2011, pp. 551–568. https://doi.org/10.5772/21790.
Shah, S. R., B. T. Smith, A. M. Tatara, E. R. Molina, E. J. Lee, T. C. Piepergerdes, B. A. Uhrig, R. E. Guldberg, G. N. Bennett, J. C. Wenke, and A. G. Mikos. Effects of local antibiotic delivery from porous space maintainers on infection clearance and induction of an osteogenic membrane in an infected bone defect. Tissue Eng. A 23:91–100, 2017.
Sharkawy, A. A., B. Klitzman, G. A. Truskey, and W. M. Reichert. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J. Biomed. Mater. Res. 37:401–412, 1997.
Taylor, B. C., B. G. French, T. T. Fowler, J. Russell, and A. Poka. Induced membrane technique for reconstruction to manage bone loss. J. Am. Acad. Orthop. Surg. 20:142–150, 2012.
Taylor, B. C., J. Hancock, R. Zitzke, and J. Castaneda. Treatment of bone loss with the induced membrane technique: techniques and outcomes. J. Orthop. Trauma 29:554–557, 2015.
Wang, J.-S., H. Franzen, E. Jonsson, and L. Lidgren. Porosity of bone cement reduced by mixing and collecting under vacuum. Acta Orthop. Scand. 64:143–146, 1993.
Ward, W. K., E. P. Slobodzian, K. L. Tiekotter, and M. D. Wood. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials 23:4185–4192, 2002.
Wehner, T., K. Gruchenberg, R. Bindl, S. Recknagel, M. Steiner, A. Ignatius, and L. Claes. Temporal delimitation of the healing phases via monitoring of fracture callus stiffness in rats. J. Orthop. Res. 32:1589–1595, 2014.
Wixson, R. L., E. P. Lautenschlager, and M. A. Novak. Vacuum mixing of acrylic bone cement. J. Arthroplast. 2:141–149, 1987.
Yang, X., B. F. Ricciardi, A. Hernandez-Soria, Y. Shi, N. Pleshko Camacho, and M. P. G. Bostrom. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41:928–936, 2007.
Acknowledgments
We would like to thank Brendon King and Stephanie Podgorny for their efforts on these projects as part of the STARS Summer Program for High School Students (data collection). This work was supported by the Washington University Musculoskeletal Research Center (NIH P30 AR057235) as well as direct funding from the AO Foundation (AO Start-up Grant S-15-190M) and Saint Louis University (Presidential Research Fund).
Conflict of interest
Dr. J. Tracy Watson has intellectual property rights with and receives royalties from Smith and Nephew, Zimmer Biomet, and Advanced Orthopaedic Solutions. He has intellectual property rights with and is a Consultant for Advanced Orthopaedic Solutions. None of these are direct conflicts of interest to this research. All other authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Toth, Z., Roi, M., Evans, E. et al. Masquelet Technique: Effects of Spacer Material and Micro-topography on Factor Expression and Bone Regeneration. Ann Biomed Eng 47, 174–189 (2019). https://doi.org/10.1007/s10439-018-02137-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-02137-5