Abstract
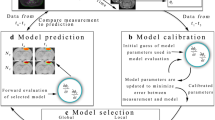
The spatiotemporal variations in tumor vasculature inevitably alters cell proliferation and treatment efficacy. Thus, rigorous characterization of tumor dynamics must include a description of this phenomenon. We have developed a family of biophysical models of tumor growth and angiogenesis that are calibrated with diffusion-weighted magnetic resonance imaging (DW-MRI) and dynamic contrast-enhanced (DCE-) MRI data to provide individualized tumor growth forecasts. Tumor and blood volume fractions were evolved using two, coupled partial differential equations consisting of proliferation, diffusion, and death terms. To evaluate these models, rats (n = 8) with C6 gliomas were imaged seven times. The tumor volume fraction was estimated using DW-MRI, while DCE-MRI provided estimates of the blood volume fraction. The first three time points were used to calibrate model parameters, which were then used to predict growth at the remaining four time points and compared directly to the measurements. The best performing model predicted tumor growth with less than 10.3% error in tumor volume and with less than 9.4% error at the voxel-level at all prediction time points. The best performing model resulted in less than 9.3% error in blood volume at the voxel-level. This pre-clinical study demonstrates the potential for image-based, mechanistic modeling of tumor growth and angiogenesis.




Similar content being viewed by others
References
Akaike, H. A new look at the statistical model identification. New York: Springer, 1974.
Anderson, A. R. A., and M. A. J. Chaplain. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull. Math. Biol. 60:857–899, 1998.
Barnes, S. L., A. G. Sorace, M. E. Loveless, J. G. Whisenant, and T. E. Yankeelov. Correlation of tumor characteristics derived from DCE-MRI and DW-MRI with histology in murine models of breast cancer. NMR Biomed. 1:1–2, 2015. https://doi.org/10.1002/nbm.3377.
Barnes, S. L., A. G. Sorace, J. G. Whisenant, J. O. McIntyre, H. Kang, and T. E. Yankeelov. DCE- and DW-MRI as early imaging biomarkers of treatment response in a preclinical model of triple negative breast cancer. NMR Biomed. 30:e3799, 2017.
Burger, M., M. Di Francesco, J. Pietschmann, and B. Schlake. Nonlinear cross-diffusion with size exclusion. SIAM J. Math. Anal. 42:2842–2871, 2010.
Cheng, H.-L. M., C. Wallis, Z. Shou, and W. A. Farhat. Quantifying angiogenesis in VEGF-enhanced tissue-engineered bladder constructs by dynamic contrast-enhanced MRI using contrast agents of different molecular weights. J. Magn. Reson. Imaging 25:137–145, 2007.
Deisboeck, T. S., and Z. Wang. Cancer dissemination: a consequence of limited carrying capacity? Med. Hypotheses 69:173–177, 2007.
Garg, I., and M. I. Miga. Preliminary investigation of the inhibitory effects of mechanical stress in tumor growth. Bellingham: International Society for Optics and Photonics, 2008.
Gevertz, J. L., and S. Torquato. Modeling the effects of vasculature evolution on early brain tumor growth. J. Theor. Biol. 243:517–531, 2006.
Gillies, R. J., P. A. Schornack, T. W. Secomb, and N. Raghunand. Causes and effects of heterogeneous perfusion in tumors. Neoplasia 1:197–207, 1999.
Helmlinger, G., P. A. Netti, H. C. Lichtenbeld, R. J. Melder, and R. K. Jain. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotech. 15:778–783, 1997.
Hormuth II, D., S. B. Eldridge, J. Weis, M. I. Miga, and T. E. Yankeelov. Mechanically coupled reaction-diffusion model to predict glioma growth: methodological details. In: Springer Methods and Protocols: Cancer Systems Biology, edited by L. von Stechow. New York: Springer, pp. 225–241, 2018. https://doi.org/10.1007/978-1-4939-7493-1_11
Hormuth, II, D. A., J. T. Skinner, M. D. Does, and T. E. Yankeelov. A comparison of individual and population-derived vascular input functions for quantitative DCE-MRI in rats. Magn. Reson. Imaging 32:397–401, 2014.
Hormuth, D. A., J. A. Weis, S. Barnes, M. I. Miga, V. Quaranta, and T. E. Yankeelov. Biophysical modeling of in vivo glioma response after whole-brain radiation therapy in a murine model of brain cancer. Int. J. Radiat. Oncol. 100:1270–1279, 2018.
Hormuth, II, D. A., J. A. Weis, S. L. Barnes, M. I. Miga, E. C. Rericha, V. Quaranta, and T. E. Yankeelov. A mechanically-coupled reaction-diffusion model that incorporates intra-tumoral heterogeneity to predict in vivo glioma growth. J. R. Soc. Interface 14:20161010, 2017.
Huston III, J. Magnetic resonance elastography of the brain. In: Magnetic Resonance Elastography SE - 8. New York: Springer, pp. 89–98, 2014.
Jain, R. K., E. di Tomaso, D. G. Duda, J. S. Loeffler, A. G. Sorensen, and T. T. Batchelor. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 8:610–622, 2007.
Jarrett, A., D. Hormuth, II, S. Barnes, X. Feng, W. Huang, and T. Yankeelov. Incorporating drug delivery into an imaging-driven, mechanics-coupled reaction diffusion model for predicting the response of breast cancer to neoadjuvant chemotherapy: theory and preliminary clinical results. Phys. Med. Biol. 63:105015, 2018.
Neal, M. L., A. D. Trister, T. Cloke, R. Sodt, S. Ahn, A. L. Baldock, C. A. Bridge, A. Lai, T. F. Cloughesy, M. M. Mrugala, J. K. Rockhill, R. C. Rockne, and K. R. Swanson. Discriminating survival outcomes in patients with glioblastoma using a simulation-based. Patient-specific response metric. PLoS ONE 8:e51951, 2013.
O’Connor, J. P. B., A. Jackson, G. J. M. Parker, C. Roberts, and G. C. Jayson. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat. Rev. Clin. Oncol. 9:167–177, 2012.
Padhani, A. R., G. Liu, D. Mu-Koh, T. L. Chenevert, H. C. Thoeny, T. Takahara, A. Dzik-Jurasz, B. D. Ross, M. Van Cauteren, D. Collins, D. A. Hammoud, G. J. S. Rustin, B. Taouli, and P. L. Choyke. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125, 2009.
Rong, Y., D. L. Durden, E. G. Van Meir, and D. J. Brat. “Pseudopalisading” necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 65:529–539, 2006.
Roque, T., L. Risser, V. Kersemans, S. Smart, D. Allen, P. Kinchesh, S. Gilchrist, A. L. Gomes, J. A. Schnabel, and M. A. Chappell. A DCE-MRI Driven 3-D reaction-diffusion model of solid tumour growth. IEEE Trans. Med. Imaging 1, 2017
Sobol′, I. M. Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math. Comput. Simul. 55:271–280, 2001.
Sourbron, S. P., and D. L. Buckley. Classic models for dynamic contrast-enhanced MRI. NMR Biomed. 2013. https://doi.org/10.1002/nbm.2940.
Stamper, I. J., H. M. Byrne, M. R. Owen, and P. K. Maini. Modelling the role of angiogenesis and vasculogenesis in solid tumour growth. Bull. Math. Biol. 69:2737–2772, 2007.
Sugahara, T., Y. Korogi, M. Kochi, I. Ikushima, Y. Shigematu, T. Hirai, T. Okuda, L. Liang, Y. Ge, Y. Komohara, Y. Ushio, and M. Takahashi. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 9:53–60, 1999.
Swanson, K. R., R. C. Rockne, J. Claridge, M. A. Chaplain, E. C. Alvord, and A. R. A. Anderson. Quantifying the role of angiogenesis in malignant progression of gliomas: in silico modeling integrates imaging and histology. Cancer Res. 71:7366–7375, 2011.
Weis, J. A., M. I. Miga, L. R. Arlinghaus, X. Li, V. Abramson, A. B. Chakravarthy, P. Pendyala, and T. E. Yankeelov. Predicting the response of breast cancer to neoadjuvant therapy using a mechanically coupled reaction-diffusion model. Cancer Res. 2015. https://doi.org/10.1158/0008-5472.can-14-2945.
Yankeelov, T. E., R. G. Abramson, and C. C. Quarles. Quantitative multimodality imaging in cancer research and therapy. Nat. Rev. Clin. Oncol. 11:670–680, 2014.
Yankeelov, T. E., and J. C. Gore. Dynamic contrast enhanced magnetic resonance imaging in oncology: theory, data acquisition, analysis, and examples. Curr. Med. Imaging Rev. 3:91–107, 2009.
Yankeelov, T. E., V. Quaranta, K. J. Evans, and E. C. Rericha. Toward a science of tumor forecasting for clinical oncology. Cancer Res. 75:918–923, 2015.
Acknowledgments
The authors acknowledge the Texas Advanced Computing Center (TACC) for providing computing resources. This work was supported through funding from the National Cancer Institute R01CA138599 and U01CA174706, CPRIT RR160005, and AAPM Research Seed Funding.
Funding
Grant Sponsor: NCI U01 CA174706, NCI U01 CA154602, CPRIT RR160005, AAPM Research Seed Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hormuth, D.A., Jarrett, A.M., Feng, X. et al. Calibrating a Predictive Model of Tumor Growth and Angiogenesis with Quantitative MRI. Ann Biomed Eng 47, 1539–1551 (2019). https://doi.org/10.1007/s10439-019-02262-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02262-9