Abstract
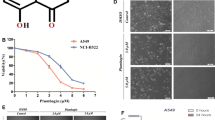
This report is designed to explore the roles of caspase-8, -9 and -3 in artemisinin (ARTE)-induced apoptosis in non-small cell lung cancer cells (A549 cells). ARTE induced reactive oxygen species (ROS)-mediated apoptosis in dose- and time-dependent fashion. Although ARTE treatment did not induce Bid cleavage and significant loss of mitochondrial membrane potential, it induced release of Smac and AIF but not cytochrome c from mitochondria, and silencing of Bak but not Bax significantly prevented ARTE-induced cytotoxicity. Moreover, ARTE treatment induced ROS-dependent activation of caspase-9, -8 and -3. Of the utmost importance, silencing or inhibiting any one of caspase-8, -9 and -3 almost completely prevented ARTE-induced activation of all the three caspases and remarkably abrogated the cytotoxicity of ARTE, suggesting that ARTE triggered an amplification activation loop among caspase-9, -8 and -3. Collectively, our data demonstrate that ARTE induces a ROS-mediated amplification activation loop among caspase-9, -8 and -3 to dominantly mediate the apoptosis of A549 cells.






Similar content being viewed by others
References
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18:767–773
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE 2:1–8
Sunder SN, Marconett CN, Doan VB, Willoughby JA Sr, Firestone GL (2008) Artemisinin selectively decreases functional levels of estrogen receptor-alpha and ablates estrogen-induced proliferation in human breast cancer cells. Carcinogenesis 29:2252–2258
Willoughby JA Sr, Sundar SN, Cheung M, Tin AS, Modiano J et al (2009) Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting sp1 interactions with the cyclin-dependent kinase-4 promoter and inhibiting CDK4 gene expression. J Biol Chem 284:2203–2213
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70:49–56
Handrick R, Ontikatze T, Bauer KD, Freier F, Rubel A et al (2010) Dihydroartemisinin induces apoptosis by a Bak-dependent intrinsic pathway. Mol Cancer Ther 9:2497–2510
Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J et al (2007) Evidence for the involvement of carbon-centered radials in the induction of apoptotic cell death by artemisinin compounds. J Biol Chem 282:9372–9382
Mercer AE, Copple IM, Maggs JL, O’Neill PM, Park BK (2011) The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J Biol Chem 286:987–996
Hou JM, Wang DS, Zhang RW, Wang H (2008) Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 14:5519–5530
Lu YY, Chen TS, Qu JL, Pan WL, Sun L, Wei XB (2009) Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma cells. J Biomed Sci 16:1–16
Lu YY, Chen TS, Wang XP, Li L (2010) Single-cell analysis of dihydroartemisinin-induced apoptosis through reactive oxygen species-mediated caspase-8 activation and mitochondrial pathway in ASTC-a-1 cells using fluorescence imaging techniques. J Biomed Opt 15:046028
Lu YY, Chen TS, Wang XP, Qu JL, Chen M (2010) The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett 584:4019–4026
Xiao FL, Gao WJ, Wang XP, Chen TS (2012) Amplification activation loop between caspase-8 and -9 dominates artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis 17:600–611
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu Rev Biochem 68:383–424
Li P, Nijhawan D, Wang XD (2004) Mitochondrial activation of apoptosis. Cell 116:S57–S59
McDonnell MA, Wang D, Khan SM, Heiden MGV, Kelekar A (2003) Caspase-9 is activated in a cytochrome c-independent manner early during TNF-α-induced apoptosis in murine cells. Cell Death Differ 10:1005–1015
Chandra D, Choy G, Deng XD, Bhatia B, Daniel P, Tang DG (2004) Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol 24:6592–6607
Shelton SN, Shawgo ME, Robertson JD (2009) Cleavage of Bid by executioner caspases mediates feed forward amplification of mitochondrial outer membrane permeabilization during genotoxic stress-induced apoptosis in Jurkat cells. J Biol Chem 284:11247–11255
Ray R, Simbulan-Rosenthal CM, Keyser BM, Benton B, Anderson D et al (2010) Sulfur mustard induces apoptosis in lung epithelial cells via a caspase amplification loop. Toxicology 271:94–99
Pan JX, Xu GP, Yeung SC (2001) Cytochrome c release is upstream to activation of caspase-9, caspase-8, and caspase-3 in the enhanced apoptosis of Anaplastic Thyroid Cancer cells induced by manumycin and paclitaxel. J Clin Endocrinol Metab 86:4731–4740
Sitailo LA, Tibudan SS, Denning MF (2002) Activation of caspase-9 is required for UV-induced apoptosis of human Keratinocytes. J Biol Chem 277:19346–19352
Hippe D, Lytovchenko O, Schmitz I, Lüder CGK (2008) Fas/CD95-mediated apoptosis of type II cells is blocked by Toxoplasma gondii primarily via interference with the mitochondrial amplification loop. Infect Immun 76:2905–2912
Metkar SS, Wang B, Ebbs ML, Kim JH, Lee YJ et al (2003) Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J Cell Biol 160(6):875–885
Wang L, Du FH, Wang XD (2008) TNF-alpha induces two distinct caspase-8 activation pathways. Cell 133:693–703
Gyrd-Hansen M, Farkas T, Fehrenbacher N, Bastholm L, Høyer-Hansen M et al (2006) Apoptosome-independent activation of the lysosomal cell death pathway by caspase-9. Mol Cell Biol 26:7880–7891
Day TW, Wu CH, Safa AR (2009) Etoposide induces protein kinase Cδ- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol Pharmacol 76:632–640
Minichsdorfer C, Hohenegger M (2009) Autocrine amplification loop in statin-induced apoptosis of human melanoma cells. Brit J Pharmacol 157:1278–1290
Zhang WW, Wang XP, Chen TS (2012) Resveratrol induces apoptosis via a Bak-mediated intrinsic pathway in human lung adenocarcinoma cells. Cell Signal 24:1037–1046
Wu YY, Xing D, Chen WR, Wang XC (2007) Bid is not required for Bax translocation during UV-induced apoptosis. Cell Signal 19:2468–2478
Düssmann H, Rehm M, Concannon CG, Anguissola S, Würstle M (2010) Single-cell quantification of Bax activation and mathematical modelling suggest pore formation on minimal mitochondrial Bax accumulation. Cell Death Differ 17:278–290
Chen T, Li M, Zhang R, Wang H (2009) Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med 13:1358–1370
Mu DG, Zhang W, Chu DL, Liu TG, Xie YH et al (2008) The role of calcium, P38 MAPK in dihydroartemisinin-induced apoptosis of lung cancer PC-14 cells. Cancer Chemother Pharm 61:639–645
Youns M, Efferth T, Reichling J, Fellenberg K, Bauer A, Hoheisel JD (2009) Gene expression profiling identifies novel key players involved in the cytotoxic effect of artesunate on pancreatic cancer cells. Biochem Pharmacol 78:273–283
Michaelis M, Kleinschmidt MC, Barth S, Rothweiler F, Geiler J et al (2010) Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem Pharmacol 79:130–136
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R et al (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem 286:6587–6601
Zhou CJ, Pan WL, Wang XP, Chen TS (2012) Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells. J Cell Physiol 227:3778–3786
Li SH, Xue F, Cheng ZY, Yang XY, Wang SY et al (2009) Effect of artesunate on inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NFκB p65. Int J Hematol 9(4):513–521
Nam W, Tak J, Ryu JK, Jung M, Yook JI et al (2007) Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck J Sci Spec 29:335–340
Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E et al (2009) Transferrin receptor- dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett 274:290–298
Stockwin LH, Han BN, Yu SX, Hollingshead MG, ElSohly MA et al (2009) Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int J Cancer 125:1266–1275
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27:6434–6451
Wang XD (2001) The expanding role of mitochondria in apoptosis. Gene Dev 15:2922–2933
Acknowledgments
We thank Dr. K. Taira for providing CFP-Bid plasmid and Dr. Y. Gotoh for providing DsRed-Mito plasmid. This work was supported by National Natural Science Foundation of China (31071218, 61178078 and 81071491) and Key Project of the Department of Education and Finance of Guangdong Province (cxzd115).
Author information
Authors and Affiliations
Corresponding author
Additional information
W. Gao and F. Xiao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, W., Xiao, F., Wang, X. et al. Artemisinin induces A549 cell apoptosis dominantly via a reactive oxygen species-mediated amplification activation loop among caspase-9, -8 and -3. Apoptosis 18, 1201–1213 (2013). https://doi.org/10.1007/s10495-013-0857-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0857-z