Abstract
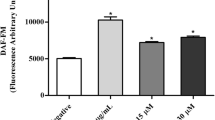
Leishmaniasis is a neglected tropical disease that affects millions of people worldwide. Current therapies mainly rely on antimonial drugs that are inadequate because of their high toxicity and increased drug resistance. An urgent need exists to discover new, more effective, more affordable, and more target-specific drugs. Pathways that are associated with apoptosis-like cell death have been identified in unicellular eukaryotes, including protozoan parasites. In the present study, we studied the mechanism of cell death that is induced by A3K2A3 against L. amazonensis. A3K2A3 is a dibenzylideneacetone that has an acyclic dienone that is attached to aryl groups in both β-positions, which is similar to curcuminoids and chalcone structures. This compound was previously shown to be safe with regard to cytotoxicity and active against the parasite. Biochemical and morphological approaches were used in the present study. The results suggested that A3K2A3 caused mitochondrial dysfunction in L. amazonensis promastigotes, leading to mechanisms of cell death that share some common phenotypic features with metazoan apoptosis, such as an increase in reactive oxygen species production, a decrease in the adenosine triphosphate ratio, phosphatidylserine exposure, a decrease in cell volume, caspase production, and DNA fragmentation. Altogether, these findings indicate that apoptosis can indeed be triggered by chemotherapeutic agents.














Similar content being viewed by others
References
Rajasekaran R, Chen Yi-Ping P (2015) Potential therapeutic targets and the role of technology in developing novel antileishmanial drugs. Drug Discov Today 20(8):958–968. doi:10.1016/j.drudis.2015.04.006
Duszenko M, Figarella K, Macleod ET, Welburn SC (2006) Death of a trypanosome: a selfish altruism. Trends Parasitol 22(11):536–542. doi:10.1016/j.pt.2006.08.010
Arambage SC, Grant KM, Pardo I, Ranford-Cartwright L, Hurd H. (2009) Malaria ookinetes exhibit multiple markers for apoptosis-like programmed cell death in vitro. Parasit Vector 2(1):32. doi:10.1186/1756-3305-2-32
Kathuria M, Bhattacharjee A, Sashidhara KV, Singh SP, Mitra K (2014) Induction of mitochondrial dysfunction and oxidative stress in Leishmania donovani by orally active clerodane diterpene. Antimicrob Agents Chemother 58(10):5916–5928. doi:10.1128/AAC.02459-14
Din ZU, Fill TP, de Assis FF, Lazarin-Bidóia D, Kaplum V, Garcia FP, Nakamura CV, Oliveira KT, Rodrigues-Filho E (2014) Unsymmetrical 1, 5-diaryl-3-oxo-1, 4-pentadienyls and their evaluation as antiparasitic agents. Bioorg Med Chem 22(3):1121–1127. doi:10.1016/j.bmc.2013.12.020
Shi M, Cai Q, Yao L, Mao Y, Ming Y, Ouyang G (2006) Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol Int 30(3):221–226. doi:10.1016/j.cellbi.2005.10.024
Aher RB, Wanare G, Kawathekar N, Kumar RR, Kaushik NK, Sahal D, Chauhan VS (2011) Dibenzylideneacetone analogues as novel Plasmodium falciparum inhibitors. Bioorg Med Chem Lett 21(10):3034–3036. doi:10.1016/j.bmcl.2011.03.037
Prakobwong S, Gupta SC, Kim JH, Sung B, Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S, Aggarwal BB (2011) Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 32(9):1372–1380. doi:10.1093/carcin/bgr032
Prakobwong S, Khoontawad J, Yongvanit P, Pairojkul C, Hiraku Y, Sithithaworn P, Pinlaor P, Aggarwa BB, Pinlaor S (2011) Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer 129(1):88–100. doi:10.1002/ijc.25656
Yu HJ, Shin JA, Nam JS, Kang BS, Cho SD (2013) Apoptotic effect of dibenzylideneacetone on oral cancer cells via modulation of specificity protein 1 and Bax. Oral Dis 19(8):767–774. doi:10.1111/odi.12062
Prasad S, Yadav VR, Ravindran J, Aggarwal BB (2011) ROS and CHOP are critical for dibenzylideneacetone to sensitize tumor cells to TRAIL through induction of death receptors and downregulation of cell survival proteins. Cancer Res 71(2):538–549. doi:10.1158/0008-5472
Britta EA, Scariot DB, Falzirolli H, Ueda-Nakamura T, Silva CC, Dias Filho BP, Borsali R, Nakamura CV (2014) Cell death and ultrastructural alterations in Leishmania amazonensis caused by new compound 4-Nitrobenzaldehyde thiosemicarbazone derived from S-limonene. BMC Microbiol 14(1):236. doi:10.1186/s12866-014-0236-0
Rodrigues JH da S, Ueda-Nakamura T, Corrêa AG, Sangi DP, Nakamura CV (2014) A quinoxaline derivative as a potent chemotherapeutic agent, alone or in combination with benznidazole, against Trypanosoma cruzi. PLoS One 9(1):e85706. doi:10.1371/journal.pone.0085706
Menna-Barreto RF, Goncalves RL, Costa EM, Silva RS, Pinto AV, Oliveira MF, de Castro SL (2009) The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radical Bio Med 47(5):644–653. doi:10.1016/j.freeradbiomed.2009.06.004
Shukla AK, Patra S, Dubey VK (2012) Iridoid glucosides from Nyctanthes arbortristis result in increased reactive oxygen species and cellular redox homeostasis imbalance in Leishmania parasite. Eur J Med Chem 54:49–58. doi:10.1016/j.ejmech.2012.04.034
Takahashi M, Shibata M, Niki E (2001) Estimation of lipid peroxidation of live cells using a fluorescent probe, diphenyl-1-pyrenylphosphine. Free Radical Bio Med 31(2):164–174. doi:10.1016/S0891-5849(01)00575-5
Lazarin-Bidóia D, Desoti VC, Ueda-Nakamura T, Dias Filho BP, Nakamura CV, Silva SO (2013) Further evidence of the trypanocidal action of eupomatenoid-5: confirmation of involvement of reactive oxygen species and mitochondria owing to a reduction in trypanothione reductase activity. Free Radical Bio Med 60:17–28. doi:10.1016/j.freeradbiomed.2013.01.008
Britta EA, Silva AB, Ueda-Nakamura T, Dias-Filho BP, Silva CC, Sernaglia RL, Nakamura CV (2012) Benzaldehyde thiosemicarbazone derived from limonene complexed with copper induced mitochondrial dysfunction in Leishmania amazonensis. PLOS One 7(8):1–12. doi:10.1371/journal.pone.0041440
Stefanello TF, Panice MR, Ueda-Nakamura T, Sarragiotto MH, Auzély-Velty R, Nakamura CV (2014) N-butyl-[1-(4-Methoxy)phenyl-9H–carboline]–3-carboxamide prevents cytokinesis in Leishmania amazonensis. Antimicrob Agents Chemother 58(12):7112–7120. doi:10.1128/AAC.03340-14
Jimenez V, Paredes R, Sosa MA, Galanti N (2008) Natural programmed cell death in T. cruzi epimastigotes maintained in axenic cultures. J Cell Biochem 105(3):688–698. doi:10.1002/jcb.21864
Kaur J, Singh BK, Tripathi RP, Singh P, Singh N (2009) Leishmania donovani: a glycosyl dihydropyridine analogue induces apoptosis like cell death via targeting pteridine reductase 1 in promastigotes. Exp Parasitol 123:258–264. doi:10.1016/j.exppara.2009.07.009
Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, Rakshit S, Sen T, Majumder HK (2004) Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ 11(8):924–936. doi:10.1038/sj.cdd.4401435
Arnoult D, Akarid K, Grodet A, Petit PX, Estaquier J, Ameisen JC (2002) On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ 9:65–81. doi:10.1038/sj.cdd.4400951
Kulkarni MM, McMaster WR, Kamysz W, McGwire BS (2009) Antimicrobial peptide-induced apoptotic death of Leishmania results from calcium-dependent, caspase-independent mitochondrial toxicity. J Biol Chem 284(23):15496–15504. doi:10.1074/jbc.M809079200
Goto H, Lindoso, JAL (2012) Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin N Am 26(2):293–307. doi:10.1016/j.idc.2012.03.001
Bhandarkar SS, Bromberg J, Carrillo C, Selvakumar P, Sharma RK, Perry BN, Govindarajan B, Fried L, Sohn A, Reddy K, Arbiser JL (2008) Tris (dibenzylideneacetone) dipalladium, a N-myristoyltransferase-1 inhibitor, is effective against melanoma growth In vitro and In vivo. Clin Cancer Res 14(18):5743–5748. doi:10.1158/1078-0432.CCR-08-0405
Vendrametto MC, Santos AO, Nakamura CV, Dias Filho BP, Cortez DAG, Ueda-Nakamura T (2010) Evaluation of antileishmanial activity of eupomatenoid-5, a compound isolated from leaves of Piper regnellii var. pallescens. Parasitol Int 59(2):154–158. doi:10.1016/j.parint.2009.12.009
Fernandes-Rodrigues JC, Souza WD (2008) Ultrastructural alterations in organelles of parasitic protozoa induced by different classes of metabolic inhibitors. Curr Pharm Des 14(9):925–938. doi:10.2174/138161208784041033
Kafetzis DA, Velissariou IM, Stabouli S, Mavrikou M, Delis D, Liapi G (2005) Treatment of paediatric visceral leishmaniasis: amphotericin B or pentavalent antimony compounds? Int J Antimicrob Agents 25:26–30. doi:10.1016/j.ijantimicag.2004.09.01131
Tiuman TS, Ueda-Nakamura T, Cortez DAG, Dias-Filho BP, Morgado-Díaz JA, Souza W, Nakamura CV (2005) Antileishmanial activity of parthenolide, a sesquiterpene lactona isolated from Tanacetum parthenium. Antimicrob Agents Chemother 49:176–182. doi:10.1128/AAC.49.11.176-182.2005
Ueda-Nakamura T, Mendonça-Filho RR, Morgado-Díaz JA, Maza PK, Dias-Filho BP, Cortez DAG et al (2006) Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol Int 55(2):99–105. doi:10.1016/j.parint.2005.10.006
Inácio JDF, Canto-Cavalheiro MM, Menna-Barreto RFS, Almeida-Amaral EE (2012) Mitochondrial damage contribute to epigallocatechin-3-gallate induced death in Leishmania amazonensis. Exp Parasitol 132(2):151–155. doi:10.1016/j.exppara.2012.06.008
Medina JM, Rodrigues JCF, De Souza W, Atella GC, Barrabim H (2012) Tomatidine promotes the inhibition of 24-alkylated sterol biosynthesis and mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitology 139(10):1253–1265. doi:10.1017/S0031182012000522
Bringaud F, Rivière L, Coustou V (2006) Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasite 149:1–9. doi:10.1016/j.molbiopara.2006.03.017
Sen N, Das BB, Ganguly A, Mukherjee T, Bandyopadhyay S, Majumder HK (2004) Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J Biol Chem 279(50):52366–52375. doi:10.1074/jbc.M406705200
Mukherjee SB, Das M, Sudhandiran G, Shaha C (2002) Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J Biol Chem 277(27):24717–24727. doi:10.1074/jbc.M201961200
Chowdhury S, Mukherjee T, Chowdhury SR, Sengupta S, Mukhopadhyay S, Jaisankar P, Majumder HK (2014) Disuccinyl betulin triggers metacaspase-dependent endonuclease G-mediated cell death in unicellular protozoan parasite Leishmania donovani. Antimicrob Agents Chemother 58(4):2186–2201. doi:10.1128/AAC.02193-13
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radical Bio Med 47(4):333–343. doi:10.1016/j.freeradbiomed.2009.05.004
Smirlis D, Duszenko M, Ruiz AJ, Scoulica E, Bastien P, Fasel N, Soteriadou K (2010) Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasite Vector 3:107. doi:10.1186/1756-3305-3-107
Fonseca-Silva F, Inacio JD, Canto-Cavalheiro MM, Almeida-Amaral EE (2011) Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One 6(2):e14666. doi:10.1371/journal.pone.0014666
Islamuddin M, Chouhan G, Tyagi M, Abdin MZ, Sahal D, Afrin F (2014) Leishmanicidal activities of Artemisia annua leaf essential oil against visceral leishmaniasis. Front Microbiol 5:626. doi:10.3389/fmicb.2014.00626
Mehta A, Shaha C (2006) Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species. Ca2+ and glutathione. Free Radical Bio Med 40:1857–1868. doi:10.1016/j.freeradbiomed.2006.01.024
Mandal G, Wyllie S, Singh N, Sundar S, Fairlamb AH, Chatterjee M (2007) Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology 134(12):1679–1687. doi:10.1017/S0031182007003150
Sarkar A, Mandal G, Singh N, Sundar S, Chatterjee M (2009) Flow cytometric determination of intracellular non-protein thiols in Leishmania promastigotes using 5-chloromethyl fluorescein diacetate. Exp Parasitol 122(4):299–305. doi:10.1016/j.exppara.2009.04.012
Koonin EV, Aravind L (2002) Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ 9(4):394–404. doi:10.1038/sj/cdd/4400991
Mehta A, Shaha C (2004) Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition complex II inhibition results in increased pentamidine citotoxicity. J Biol Chem 279(12):11798–11813. doi:10.1074/jbc.M309341200
Dutta A, Ghoshal A, Mandal D, Mondal NB, Banerjee S, Sahu NP, Mandal C (2007) Racemoside A, an anti-leishmanial, water-soluble, natural steroidal saponin, induces programmed cell death in Leishmania donovani. J Med Microbiol 56(9):1196–1204. doi:10.1099/jmm.0.47114-0
Kroemer G, Galluzzi L, Vandenabeele P et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16(1):3–11. doi:10.1038/cdd.2008.150
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2(4):277–288. doi:10.1038/nrc776
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9(1):49–89. doi:10.1089/ars.2007.9.49
Facompre M, Goossens JF, Bailly C (2001) Apoptotic response of HL-60 human leukemia cells to the antitumor drug NB-506, a glycosylated indolocarbazole inhibitor of topoisomerase 1. Biochem Pharmacol 61(3):299–310. doi:10.1016/S0006-2952(00)00553-0
Kosec G, Alvarez VE, Agüero F, Sánchez D, Dolinar M, Turk B, Turk V, Cazzulo JJ (2006) Metacaspases of Trypanosoma cruzi: possible candidates for programmed cell death mediators. Mol Biochem Parasitol 145:18–28. doi:10.1016/j.molbiopara.2005.09.001
Lee N, Gannavaram S, Selvapandiyan A, Debrabant A (2007) Characterization of metacaspases with trypsin-like activity and their putative role in programmed cell death in the protozoan parasite Leishmania. Eukaryot Cell 6:1745–1757. doi:10.1128/EC.00123-07
Casanova M, Gonzalez IJ, Sprissler C, Zalila H, Dacher M, Basmaciyan L, Späth GF, Azas N, Fasel N (2015) Implication of different domains of the Leishmania major metacaspase in cell death and autophagy. Cell Death Dis 6(10):e1933. doi:10.1038/cddis.2015.288
Martinvalet D, Zhu P, Lieberman J (2005) Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 22(3):355–370. doi:10.1016/j.immuni.2005.02.004
Zangger H, Mottram JC, Fasel N (2002) Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ 9(10):1126–1139. doi:10.1038/sj.cdd.4401071
Acknowledgments
This work was supported through grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Capacitação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, Financiadora de Estudos e Projetos—FINEP, Programa de Pós-Graduação em Ciências Biológicas da Universidade Estadual de Maringá and Complexo de Centrais de Apoio a Pesquisa COMCAP—UEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All of the animal procedures were performed in accordance with guidelines established by the Universidade Estadual de Maringá ethical committee (Protocol No. 029/2014).
Rights and permissions
About this article
Cite this article
Garcia, F.P., Henrique da Silva Rodrigues, J., Din, Z.U. et al. A3K2A3-induced apoptotic cell death of Leishmania amazonensis occurs through caspase- and ATP-dependent mitochondrial dysfunction. Apoptosis 22, 57–71 (2017). https://doi.org/10.1007/s10495-016-1308-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1308-4