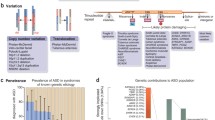
Abstract
The genetic architecture of neurodevelopmental disorders is largely polygenic, non-specific, and pleiotropic. This complex genetic architecture makes the search for specific etiological mechanisms that contribute to neurodevelopmental risk more challenging. Monogenic disorders provide an opportunity to focus in on how well-articulated signaling pathways contribute to risk for neurodevelopmental outcomes. This paper will focus on neurofibromatosis type 1 (NF1), a rare monogenic disorder that is associated with varied neurodevelopmental outcomes. Specifically, this paper will provide a brief overview of NF1 and its phenotypic associations with autism spectrum disorder, attention-deficit/hyperactivity disorder, and specific learning disorders, describe how variation within the NF1 gene increases risk for neurodevelopmental disorders via altered Ras signaling, and provide future directions for NF1 research to help elucidate the genetic architecture of neurodevelopmental disorders in the general population.

Similar content being viewed by others
References
Acosta MT (2013) Challenges of cognitive research in neurofibromatosis type 1. Lancet Neurol 12:1040–1041
Acosta MT, Gioia GA, Silva AJ (2006) Neurofibromatosis type 1: new insights into neurocognitive issues. Curr Neurol Neurosci Rep 6:136–143
Acosta MT et al (2012) The learning disabilities network (LeaDNet): using neurofibromatosis type 1 (NF1) as a paradigm for translational research. Am J Med Genet A 158A:2225–2232
Adviento B et al (2014) Autism traits in the RASopathies. J Med Genet 51:10–20
Alemany S, Jansen PR, Muetzel RL, Marques N, El Marroun H, Jaddoe VWV, Polderman TJC, Tiemeier H, Posthuma D, White T (2019) Common polygenic variations for psychiatric disorders and cognition in relation to brain morphology in the general pediatric population. J Am Acad Child Adolesc Psychiatry 58:600–607
Altarac M, Saroha E (2007) Lifetime prevalence of learning disability among US children. Pediatrics 119(Suppl 1):S77–83
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. American Psychiatric Association, Philadelphia
Anastasaki C, Gutmann DH (2014) Neuronal NF1/RAS regulation of cyclic AMP requires atypical PKC activation. Hum Mol Genet 23:6712–6721
Anastasaki C, Woo AS, Messiaen LM, Gutmann DH (2015) Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning. Hum Mol Genet 24:3518–3528
Anney R et al (2012) Individual common variants exert weak effects on the risk for autism spectrum disorders. Hum Mol Genet 21:4781–4792
Baio J et al (2018) Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 67:1–23
Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J (1992) Aberrant regulation of Ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356:713–715
Beaussart ML, Barbarot S, Mauger C, Roy A (2018) Systematic review and meta-analysis of executive functions in preschool and school-age children with neurofibromatosis type 1. J Int Neuropsychol Soc 24:977–994
Boyle EA, Li YI, Pritchard JK (2017) An expanded view of complex traits: from polygenic to omnigenic. Cell 169:1177–1186
Brain Consortium, et al. (2018). Analysis of shared heritability in common disorders of the brain. Science 360:1–15.
Brown JA, Emnett RJ, White CR, Yuede CM, Conyers SB, O'Malley KL, Wozniak DF, Gutmann DH (2010) Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Hum Mol Genet 19:4515–4528
Carithers LJ, Moore HM (2015) The genotype-tissue expression (gtex) project. Biopreserv Biobank 13:307–308
Cederlöf M, Maughan B, Larsson H, D'Onofrio BM, Plomin R (2017) Reading problems and major mental disorders - co-occurrences and familial overlaps in a Swedish nationwide cohort. J Psychiatr Res 91:124–129
Chabernaud C, Mennes M, Kardel PG, Gaillard WD, Kalbfleisch ML, Vanmeter JW, Packer RJ, Milham MP, Castellanos FX, Acosta MT (2012) Lovastatin regulates brain spontaneous low-frequency brain activity in neurofibromatosis type 1. Neurosci Lett 515:28–33
Chaix Y et al (2018) Deficit in phonological processes: a characteristic of the neuropsychological profile of children with NF1. Child Neuropsychol 24:558–574
Chisholm AK, Anderson VA, Pride NA, Malarbi S, North KN, Payne JM (2018) Social function and autism spectrum disorder in children and adults with neurofibromatosis type 1: a systematic review and meta-analysis. Neuropsychol Rev 28:317–340
Choi CH et al (2016) Multiple drug treatments that increase cAMP signaling restore long-term memory and aberrant signaling in Fragile X syndrome models. Front Behav Neurosci 10:136
Cimino PJ, Gutmann DH (2018) Neurofibromatosis type 1. Handb Clin Neurol 148:799–811
Costa RM, Silva AJ (2002) Molecular and cellular mechanisms underlying the cognitive deficits associated with neurofibromatosis 1. J Child Neurol 17:622–626; discussion 627–629, 646–651
Costa RM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI (2001) Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet 27:399–405
Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ (2002) Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415:526–530
Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ (2008) Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135:549–560
Cutting LE, Levine TM (2010) Cognitive profile of children with neurofibromatosis and reading disabilities. Child Neuropsychol 16:417–432
Cutting LE, Koth CW, Denckla MB (2000) How children with neurofibromatosis type 1 differ from "typical" learning disabled clinic attenders: nonverbal learning disabilities revisited. Dev Neuropsychol 17:29–47
Cutting LE, Cooper KL, Koth CW, Mostofsky SH, Kates WR, Denckla MB, Kaufmann WE (2002) Megalencephaly in NF1: predominantly white matter contribution and mitigation by ADHD. Neurology 59:1388–1394
Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N (1992) The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron 8:415–428
DeClue JE, Cohen BD, Lowy DR (1991) Identification and characterization of the neurofibromatosis type 1 protein product. Proc Natl Acad Sci USA 88:9914–9918
Demontis D et al (2019) Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 51:63–75
Deneault E, Faheem M, White SH, Rodrigues DC, Sun S, Wei W, Piekna A, Thompson T, Howe JL, Chalil L, Kwan V, Walker S, Pasceri P, Roth FP, Yuen RKC, Sing KK, Ellis J, Scherer SW (2019) CNTN5-/+or EHMT2-/+ human iPSC-derived neurons from individuals with Autism develop hyperactive neuronal networks. eLife 8:e40092.
Diggs-Andrews KA, Gutmann DH (2013) Modeling cognitive dysfunction in Neurofibromatosis-1. Trends Neurosci 36:237–247
Dobyns WB, Mirzaa GM (2019) Megalencephaly syndromes associated with mutations of core components of the PI3K-AKT-MTOR pathway: PIK3CA, PIK3R2, AKT3, and MTOR. Am J Med Genet C 181:582–590
Easton DF, Ponder MA, Huson SM, Ponder BA (1993) An analysis of variation in expression of neurofibromatosis (NF) type 1 (NF1): evidence for modifying genes. Am J Hum Genet 53:305–313
Eijk S et al (2018) Autism spectrum disorder in an unselected cohort of children with neurofibromatosis type 1 (NF1). J Autism Dev Disord 48:2278–2285
Endo M et al (2013) Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clin Cancer Res 19:450–461
Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F (2010) Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 152A:327–332
Faraone SV, Buitelaar J (2010) Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry 19:353–364
Faraone SV, Ghirardi L, Kuja-Halkola R, Lichtenstein P, Larsson H (2017) The familial co-aggregation of attention-deficit/hyperactivity disorder and intellectual disability: a register-based family study. J Am Acad Child Adolesc Psychiatry 56:167–174.e161
Ferner RE et al (2007) Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet 44:81–88
Finucane HK et al (2015) Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet 47:1228–1235
Fletcher SW, Wagner EH (1996) Clinical Epidemiology: The Essentials. Lippincott Williams & Wilkins, Philadelphia
Forés-Martos J et al (2019) Transcriptomic metaanalyses of autistic brains reveals shared gene expression and biological pathway abnormalities with cancer. Mol Autism 10:17
Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, Krystal JH, Murray JD, Anticevic A (2017) Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in Schizophrenia and autism spectrum disorders. Biol Psychiatry 81:848–861
Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, Green J (2013) Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population-based study. Dev Med Child Neurol 55:139–145
Garg S, Heuvelman H, Huson S, Tobin H, Green J, Network NUNR (2016) Sex bias in autism spectrum disorder in neurofibromatosis type 1. J Neurodev Disord 8:26
Ghirardi L, Brikell I, Kuja-Halkola R, Freitag CM, Franke B, Asherson P, Lichtenstein P, Larsson H (2018) The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry 23:257–262
Gonçalves J, Violante IR, Sereno J, Leitão RA, Cai Y, Abrunhosa A, Silva AP, Silva AJ, Castelo-Branco M (2017) Testing the excitation/inhibition imbalance hypothesis in a mouse model of the Autism Spectrum Disorder: in vivo neurospectroscopy and molecular evidence for regional phenotypes. Mol Autism 8:47
Gratten J, Wray NR, Keller MC, Visscher PM (2014) Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci 17:782–790
Grove J et al (2019) Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 51:431–444
Gutmann DH, Wood DL, Collins FS (1991) Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A 88:9658–9662
Gutmann DH, Zhang Y, Hirbe A (1999) Developmental regulation of a neuron-specific neurofibromatosis 1 isoform. Ann Neurol 46:777–782
Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ (2017) Neurofibromatosis type 1. Nat Rev Dis Primers 3:17004
Hawrylycz MJ et al (2012) An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489:391–399
Hoffmeyer S, Assum G, Griesser J, Kaufmann D, Nürnberg P, Krone W (1995) On unequal allelic expression of the neurofibromin gene in neurofibromatosis type 1. Hum Mol Genet 4:1267–1272
Hyman SL, Shores A, North KN (2005) The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 65:1037–1044
Insel PA, Ostrom RS (2003) Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23:305–314
Kelley DJ, Bhattacharyya A, Lahvis GP, Yin JC, Malter J, Davidson RJ (2008) The cyclic AMP phenotype of Fragile X and autism. Neurosci Biobehav Rev 32:1533–1543
Kim YS, Woo J, Lee CJ, Yoon BE (2017) Decreased glial GABA and tonic inhibition in cerebellum of mouse model for attention-deficit/hyperactivity disorder (ADHD). Exp Neurobiol 26:206–212
Klein M, van Donkelaar M, Verhoef E, Franke B (2017) Imaging genetics in neurodevelopmental psychopathology. Am J Med Genet B 174:485–537
Klein M et al (2019) Genetic markers of ADHD-related variations in intracranial volume. Am J Psychiatry 176:228–238
Koczkowska M et al (2018) Genotype-phenotype correlation in NF1: evidence for a more severe phenotype associated with missense mutations affecting NF1 codons 844–848. Am J Hum Genet 102:69–87
Koczkowska M et al (2019) Expanding the clinical phenotype of individuals with a 3-bp in-frame deletion of the NF1 gene (c.2970_2972del): an update of genotype-phenotype correlation. Genet Med 21:867–876
Koth CW, Cutting LE, Denckla MB (2000) The association of Neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol 6:185–194
Lee MJ, Stephenson DA (2007) Recent developments in neurofibromatosis type 1. Curr Opin Neurol 20:135–141
Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, Cannon TD, Silva AJ (2005) The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol 15:1961–1967
Lidzba K, Granström S, Lindenau J, Mautner VF (2012) The adverse influence of attention-deficit disorder with or without hyperactivity on cognition in neurofibromatosis type 1. Dev Med Child Neurol 54:892–897
Lion-François L et al (2014) The effect of methylphenidate on neurofibromatosis type 1: a randomised, double-blind, placebo-controlled, crossover trial. Orphanet J Rare Dis 9:142
Lundström S, Chang Z, Kerekes N, Gumpert CH, Råstam M, Gillberg C, Lichtenstein P, Anckarsäter H (2011) Autistic-like traits and their association with mental health problems in two nationwide twin cohorts of children and adults. Psychol Med 41:2423–2433
Mackay TF (2001) The genetic architecture of quantitative traits. Annu Rev Genet 35:303–339
Mainberger F, Jung NH, Zenker M, Wahlländer U, Freudenberg L, Langer S, Berweck S, Winkler T, Straube A, Heinen F, Granström S, Mautner VF, Lidzba K, Mall V (2015) Lovastatin improves impaired synaptic plasticity and phasic alertness in patients with neurofibromatosis type 1. BMC Neurol 13:131
Maloney SE, Chandler KC, Anastasaki C, Rieger MA, Gutmann DH, Dougherty JD (2018) Characterization of early communicative behavior in mouse models of neurofibromatosis type 1. Autism Res 11:44–58
Mariani J et al (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390
Mautner VF, Kluwe L, Thakker SD, Leark RA (2002) Treatment of ADHD in neurofibromatosis type 1. Dev Med Child Neurol 44:164–170
McCormick F (2015) RAS pathway v2.0. NIH. www.cancer.gov/research/key-initiatives/ras/ras-central/blog/2015/ras-pathway-v2. Accessed 11 Nov 2019
McKeever K, Shepherd CW, Crawford H, Morrison PJ (2008) An epidemiological, clinical and genetic survey of neurofibromatosis type 1 in children under sixteen years of age. Ulster Med J 77:160–163
Miller DT, Freedenberg D, Schorry E, Ullrich NJ, Viskochil D, Korf BR (2019) Health supervision for children with neurofibromatosis type 1. Pediatrics 143:e20190660
Mirzaa GM et al (2016) Association of MTOR mutations with developmental brain disorders, including megalencephaly, focal cortical dysplasia, and pigmentary mosaicism. JAMA Neurol 73:836–845
Mitra I, Lavillaureix A, Yeh E, Traglia M, Tsang K, Bearden CE, Rauen KA, Weiss LA (2017) Reverse pathway genetic approach identifies epistasis in autism spectrum disorders. PLoS Genet 13:e1006516
Moll K, Kunze S, Neuhoff N, Bruder J, Schulte-Körne G (2014) Specific learning disorder: prevalence and gender differences. PLoS ONE 9:e103537
Moore BD (2009) Potential influences on mathematical difficulties in children and adolescents with neurofibromatosis, type 1. Dev Disabil Res Rev 15:45–51
Morris SM, Gutmann DH (2018) A genotype-phenotype correlation for quantitative autistic trait burden in neurofibromatosis 1. Neurology 90:377–379
Morris SM et al (2016) Disease burden and symptom structure of autism in neurofibromatosis type 1: a study of the International NF1-ASD consortium team (INFACT). JAMA Psychiatry 73:1276–1284
National Institutes of Health (1988) National institutes of health consensus development conference statement: neurofibromatosis. Bethesda, MD, USA, July 13–15, 1987. Neurofibromatosis 1:172–178
North KN, Riccardi V, Samango-Sprouse C, Ferner R, Moore B, Legius E, Ratner N, Denckla MB (1997) Cognitive function and academic performance in neurofibrornatosis 1: Consensus statement from the NF1 cognitive disorders task force. Neurology 48:1121-1127
O'Donovan MC, Owen MJ (2016) The implications of the shared genetics of psychiatric disorders. Nat Med 22:1214–1219
Orraca-Castillo M, Estévez-Pérez N, Reigosa-Crespo V (2014) Neurocognitive profiles of learning disabled children with neurofibromatosis type 1. Front Hum Neurosci 8:386
Pantaleoni F et al (2017) Aberrant HRAS transcript processing underlies a distinctive phenotype within the RASopathy clinical spectrum. Hum Mutat 38:798–804
Payne JM, Moharir MD, Webster R, North KN (2010) Brain structure and function in neurofibromatosis type 1: current concepts and future directions. J Neurol Neurosurg Psychiatry 81:304–309
Payne JM, Hyman SL, Shores EA, North KN (2011) Assessment of executive function and attention in children with neurofibromatosis type 1: relationships between cognitive measures and real-world behavior. Child Neuropsychol 17:313–329
Payne JM et al (2016) Randomized placebo-controlled study of lovastatin in children with neurofibromatosis type 1. Neurology 87:2575–2584
Pierpont EI, Wolford M (2016) Behavioral functioning in cardiofaciocutaneous syndrome: Risk factors and impact on parenting experience. Am J Med Genet A 170:1974–1988
Pirozzi F, Nelson B, Mirzaa G (2018) From microcephaly to megalencephaly: determinants of brain size. Dialogues Clin Neurosci 20:267–282
Posthuma D, Polderman TJ (2013) What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Curr Opin Neurol 26:111–121
Reijnders MRF et al (2017) Variation in a range of mTOR-related genes associates with intracranial volume and intellectual disability. Nat Commun 8:1052
Rieley MB, Stevenson DA, Viskochil DH, Tinkle BT, Martin LJ, Schorry EK (2011) Variable expression of neurofibromatosis 1 in monozygotic twins. Am J Med Genet A 155A:478–485
Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R (2008) Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry 49:535–542
Ronald A, Edelson LR, Asherson P, Saudino KJ (2010) Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: findings from a community twin study of 2-year-olds. J Abnorm Child Psychol 38:185–196
Ronald A, Larsson H, Anckarsäter H, Lichtenstein P (2014) Symptoms of autism and ADHD: a Swedish twin study examining their overlap. J Abnorm Psychol 123:440–451
Sagata N et al (2017) Dysregulated gene expressions of MEX3D, FOS and BCL2 in human induced-neuronal (iN) cells from NF1 patients: a pilot study. Sci Rep 7:13905
Sahin M, Jones SR, Sweeney JA, Berry-Kravis E, Conners BW, Ewen JB, Hartman AL, Levin AR, Potter WZ, Mamounas LA (2018) Discovering translational biomarkers in neurodevelopmental disorders. Nat Rev Drug Discov 18:235–236
Said SM, Yeh TL, Greenwood RS, Whitt JK, Tupler LA, Krishnan KR (1996) MRI morphometric analysis and neuropsychological function in patients with neurofibromatosis. NeuroReport 7:1941–1944
Sanders SJ et al (2019) A framework for the investigation of rare genetic disorders in neuropsychiatry. Nat Med 25:1477–1487
Schafer ST et al (2019) Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci 22:243–255
Schwetye KE, Gutmann DH (2014) Cognitive and behavioral problems in children with neurofibromatosis type 1: challenges and future directions. Expert Rev Neurother 14:1139–1152
Seidlin M, Holzman R, Knight P, Korf B, Rangel Miller V, Viskochil D, Bakker A, CsT F (2017) Characterization and utilization of an international neurofibromatosis web-based, patient-entered registry: an observational study. PLoS ONE 12:e0178639
Sethna F, Feng W, Ding Q, Robison AJ, Feng Y, Wang H (2017) Enhanced expression of ADCY1 underlies aberrant neuronal signalling and behaviour in a syndromic autism model. Nat Commun 8:14359
Shofty B et al (2019) Autism-associated Nf1 deficiency disrupts corticocortical and corticostriatal functional connectivity in human and mouse. Neurobiol Dis 130:104479
Sites ER, Smolarek TA, Martin LJ, Viskochil DH, Stevenson DA, Ullrich NJ, Messiaen LM, Sokol DK, Maloney B, Westmark CJ, Lahiri DK (2019) Novel contribution of secreted amyloid-β precursor protein to white matter brain enlargement in autism spectrum disorder. Front Psychiatry 10:165
Sokol DK, Maloney B, Westmark CJ, Lahiri DK (2019) Novel contribution of secreted amyloid-β precursor protein to white matter brain enlargement in autism spectrum disorder. Front Psychia 10:165
Steen RG, Taylor JS, Langston JW, Glass JO, Brewer VR, Reddick WE, Mages R, Pivnick EK (2001) Prospective evaluation of the brain in asymptomatic children with neurofibromatosis type 1: relationship of macrocephaly to T1 relaxation changes and structural brain abnormalities. AJNR Am J Neuroradiol 22:810–817
Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ (1997) Autism and macrocephaly. Lancet 349:1744–1745
Szklarczyk D et al (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447–452
Timpson NJ, Greenwood CMT, Soranzo N, Lawson DJ, Richards JB (2018) Genetic architecture: the shape of the genetic contribution to human traits and disease. Nat Rev Genet 19:110–124
Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y (2002) Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci 5:95–96
Torres Nupan MM, Velez Van Meerbeke A, López Cabra CA, Herrera Gomez PM (2017) Cognitive and behavioral disorders in children with neurofibromatosis type 1. Front Pediatr 5:227
Trovó-Marqui AB, Tajara EH (2006) Neurofibromin: a general outlook. Clin Genet 70:1–13
Upadhyaya M, Cooper DN (1998) The mutational spectrum in neurofibromatosis 1 and its underlying mechanisms. In: Upadhyaya M, Cooper DN (eds) Neurofibromatosis type 1: from genotype to phenotype. BIOS Scientific Publishers, Oxford, pp 65–88
Uusitalo E, Leppävirta J, Koffert A, Suominen S, Vahtera J, Vahlberg T, Pöyhönen M, Peltonen J, Peltonen S (2015) Incidence and mortality of neurofibromatosis: a total population study in Finland. J Invest Dermatol 135:904–906
van der Vaart T et al (2013) Simvastatin for cognitive deficits and behavioural problems in patients with neurofibromatosis type 1 (NF1-SIMCODA): a randomised, placebo-controlled trial. Lancet Neurol 12:1076–1083
van der Voet M, Harich B, Franke B, Schenck A (2016) ADHD-associated dopamine transporter, latrophilin and neurofibromin share a dopamine-related locomotor signature in Drosophila. Mol Psychiatry 21:565–573
Van Es S, North KN, McHugh K, De Silva M (1996) MRI findings in children with neurofibromatosis type 1: a prospective study. Pediatr Radiol 26:478–487
Verhoef E et al (2019) Disentangling polygenic associations between attention-deficit/hyperactivity disorder, educational attainment, literacy and language. Transl Psychiatry 9:35
Vithayathil J, Pucilowska J, Landreth GE (2018) ERK/MAPK signaling and autism spectrum disorders. Prog Brain Res 241:63–112
Vogel AC, Gutmann DH, Morris SM (2017) Neurodevelopmental disorders in children with neurofibromatosis type 1. Dev Med Child Neurol 59:1112–1116
Walsh KS, Velez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, Castellanos FX, Acosta MT (2013) Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev Med Child Neurol 55:131–138
Watanabe K, Stringer S, Frei O, Umićević MM, de Leeuw C, Polderman TJC, van der Sluis S, Andreassen OA, Neale BM, Posthuma D (2019) Nat Genet 51:1339–1348
Watt SE, Shores A, North KN (2008) An examination of lexical and sublexical reading skills in children with Neurofibromatosis Type 1. Child Neuropsychol 14:401–418
Wegscheid ML, Anastasaki C, Gutmann DH (2018) Human stem cell modeling in neurofibromatosis type 1 (NF1). Exp Neurol 299:270–280
Willcutt EG (2012) The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9:490–499
Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF (2005) Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: a meta-analytic review. Biol Psychiatry 57:1336–1346
Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL (2009) Neurofibromatosis Type 1 revisited. Pediatrics 123:124–133
Wolman MA, de Groh ED, McBride SM, Jongens TA, Granato M, Epstein JA (2014) Modulation of cAMP and ras signaling pathways improves distinct behavioral deficits in a zebrafish model of neurofibromatosis type 1. Cell Rep 8:1265–1270
Xing X, Zhang J, Wu K, Cao B, Li X, Jiang F, Hu Z, Xia K, Li JD (2019) Suppression of Akt-mTOR pathway rescued the social behavior in Cntnap2-deficient mice. Sci Rep 9:3041
Yoncheva YN et al (2017) Computerized cognitive training for children with neurofibromatosis type 1: a pilot resting-state fMRI study. Psychiatry Res Neuroimaging 266:53–58
Zhu X, Need AC, Petrovski S, Goldstein DB (2014) One gene, many neuropsychiatric disorders: lessons from Mendelian diseases. Nat Neurosci 17:773–781
Acknowledgements
The authors would like to thank Jean Davidson for creating Figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jessica A. Kaczorowski, Taylor F. Smith, Amanda M. Shrewsbury, Leah R. Thomas, Valerie S. Knopik and Maria T. Acosta declare that they have no confict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Mark Taylor
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaczorowski, J.A., Smith, T.F., Shrewsbury, A.M. et al. Neurofibromatosis Type 1 Implicates Ras Pathways in the Genetic Architecture of Neurodevelopmental Disorders. Behav Genet 50, 191–202 (2020). https://doi.org/10.1007/s10519-020-09991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-020-09991-x