Abstract
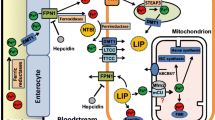
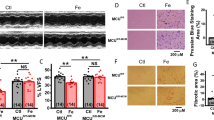
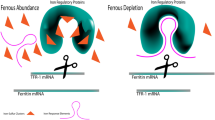
Iron-overload induced cardiomyopathy is a major cause of morbidity and mortality in thalassemic patients. Previous studies suggest that cardiac mitochondrial dysfunction may be involved in the pathogenesis of cardiomyopathy in thalassemia. We tested the hypothesis that iron overload causes dysfunction of cardiac mitochondria isolated from thalassemic mice. Cardiac mitochondria were isolated from the heart tissue of genetically-altered, β-thalassemic mice (HT) and adult wild-type mice (WT). Ferrous iron (Fe2+) at various concentrations (0–5 μg/ml) was applied to induce iron toxicity. Pharmacological interventions, facilitated by mitochondrial permeability transition pore (mPTP) blocker, CsA, and mitochondrial Ca2+ uniporter (MCU) blocker, Ru360, were used to study their respective effects on cardiac mitochondrial dysfunction. Cardiac mitochondrial ROS production, mitochondrial membrane potential changes, and mitochondrial swelling were determined. Iron overload caused increased ROS production, mitochondrial depolarization, and mitochondrial swelling in a dose-dependent manner in WT and HT cardiac mitochondria. CsA decreased only ROS production in WT and HT cardiac mitochondria, whereas Ru360 completely prevented the development of cardiac mitochondrial dysfunction by decreasing ROS, mitochondrial depolarization, and swelling in both WT and HT cardiac mitochondria. Ru360, an MCU blocker, provides protective effects by preventing ROS production and mitochondrial depolarization as well as attenuating mitochondrial swelling caused by Fe2+ overload. These findings indicate that the MCU could be a major portal for Fe2+ entry into cardiac mitochondria. Therefore, blocking MCU may be an effective therapy to prevent iron-overload induced cardiac mitochondrial dysfunction in patients with thalassemia.





Similar content being viewed by others
References
Bartfay WJ, Bartfay E (2000) Iron-overload cardiomyopathy: evidence for a free radical-mediated mechanism of injury and dysfunction in a murine model. Biol Res Nurs 2(1):49–59
Bernardi P, Forte M (2007) The mitochondrial permeability transition pore. Novartis Found Symp 287:157–164 (discussion 164–159)
Chelli B, Falleni A, Salvetti F, Gremigni V, Lucacchini A, Martini C (2001) Peripheral-type benzodiazepine receptor ligands: mitochondrial permeability transition induction in rat cardiac tissue. Biochem Pharmacol 61(6):695–705
Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG (1995) Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol 486(Pt 1):1–13
Flatmark T, Romslo I (1975) Energy-dependent accumulation of iron by isolated rat liver mitochondria. Requirement of reducing equivalents and evidence for a unidirectional flux of Fe(II) across the inner membrane. J Biol Chem 250(16):6433–6438
Forte M, Bernardi P (2005) Genetic dissection of the permeability transition pore. J Bioenerg Biomembr 37(3):121–128
Gao X, Campian JL, Qian M, Sun XF, Eaton JW (2009) Mitochondrial DNA damage in iron overload. J Biol Chem 284(8):4767–4775
Gao X, Qian M, Campian JL, Marshall J, Zhou Z, Roberts AM, Kang YJ, Prabhu SD, Sun XF, Eaton JW (2010) Mitochondrial dysfunction may explain the cardiomyopathy of chronic iron overload. Free Radic Biol Med 49(3):401–407
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117(3):285–297
Hoppe UC (2010) Mitochondrial calcium channels. FEBS Lett 584(10):1975–1981
Incharoen T, Thephinlap C, Srichairatanakool S, Chattipakorn S, Winichagoon P, Fucharoen S, Vadolas J, Chattipakorn N (2007) Heart rate variability in beta-thalassemic mice. Int J Cardiol 121(2):203–204
Jamsai D, Zaibak F, Khongnium W, Vadolas J, Voullaire L, Fowler KJ, Gazeas S, Fucharoen S, Williamson R, Ioannou PA (2005) A humanized mouse model for a common beta0-thalassemia mutation. Genomics 85(4):453–461
Jamsai D, Zaibak F, Vadolas J, Voullaire L, Fowler KJ, Gazeas S, Peters H, Fucharoen S, Williamson R, Ioannou PA (2006) A humanized BAC transgenic/knockout mouse model for HbE/beta-thalassemia. Genomics 88(3):309–315
Kim JS, He L, Lemasters JJ (2003) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304(3):463–470
Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427(6972):360–364
Kumfu S, Chattipakorn S, Srichairatanakool S, Settakorn J, Fucharoen S, Chattipakorn N (2011) T-type calcium channel as a portal of iron uptake into cardiomyocytes of beta-thalassemic mice. Eur J Haematol 86(2):156–166
Lee DW, Lee TK, Cho IS, Park HE, Jin S, Cho HJ, Kim SH, Oh S, Kim HS (2011) Creation of myocardial fibrosis by transplantation of fibroblasts primed with survival factors. Am J Physiol Heart Circ Physiol 301(3):H1004–H1014
Lekawanvijit S, Chattipakorn N (2009) Iron overload thalassemic cardiomyopathy: iron status assessment and mechanisms of mechanical and electrical disturbance due to iron toxicity. Can J Cardiol 25(4):213–218
Levi S, Rovida E (2009) The role of iron in mitochondrial function. Biochim Biophys Acta 1790(7):629–636
Novalija E, Kevin LG, Eells JT, Henry MM, Stowe DF (2003) Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology 98(5):1155–1163
Olivieri NF (1999) The beta-thalassemias. N Engl J Med 341(2):99–109
O'Rourke B, Cortassa S, Aon MA (2005) Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 20:303–315
Papanikolaou G, Pantopoulos K (2005) Iron metabolism and toxicity. Toxicol Appl Pharmacol 202(2):199–211
Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK (2010) MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467(7313):291–296
Richardson DR, Lane DJ, Becker EM, Huang ML, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P (2010) Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci USA 107(24):10775–10782
Ruiz-Meana M, Garcia-Dorado D, Miro-Casas E, Abellan A, Soler–Soler J (2006) Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res 71(4):715–724
Thummasorn S, Kumfu S, Chattipakorn S, Chattipakorn N (2011) Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion 11(3):457–466
Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ (2008) Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 48(5):1644–1654
Weatherall DJ, Clegg JB (2001) Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ 79(8):704–712
Wood JC, Enriquez C, Ghugre N, Otto-Duessel M, Aguilar M, Nelson MD, Moats R, Coates TD (2005) Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann N Y Acad Sci 1054:386–395
Yarana C, Sanit J, Chattipakorn N, Chattipakorn S (2012) Synaptic and nonsynaptic mitochondria demonstrate a different degree of calcium-induced mitochondrial dysfunction. Life Sci 90(19–20):808–814
Zorov DB, Juhaszova M, Sollott SJ (2006) Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757(5–6):509–517
Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, Di Gregorio F, Burattini MG, Terzoli S (1989) Survival and causes of death in thalassaemia major. Lancet 2(8653):27–30
Acknowledgments
We would like to thank Ms. Nusara Chomanee at the Department of Pathology, Faculty of Medicine, Siriraj Hospital for her assistance on electron microscope. This work is supported by the Thailand Research Fund Senior Research Scholar Grant (N.C), BRG 5480003 (S.C), and the Thailand Research Fund Royal Golden Jubilee PhD project (S.K and N.C).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumfu, S., Chattipakorn, S., Fucharoen, S. et al. Mitochondrial calcium uniporter blocker prevents cardiac mitochondrial dysfunction induced by iron overload in thalassemic mice. Biometals 25, 1167–1175 (2012). https://doi.org/10.1007/s10534-012-9579-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-012-9579-x