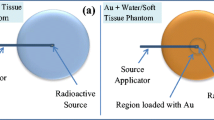
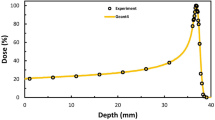
Abstract
Gold nanoparticles can enhance the biological effective dose of radiation delivered to tumors, but few data exist to quantify this effect. The purpose of this project was to build a Monte Carlo simulation model to study the degree of dose enhancement achievable with gold nanoparticles. A Monte Carlo simulation model was first built using Geant4 code. An Ir-192 brachytherapy source in a water phantom was simulated and the calculation model was first validated against previously published data. We then introduced up to 1013 gold nanospheres per cm3 into the water phantom and examined their dose enhancement effect. We compared this enhancement against a gold-water mixture model that has been previously used to attempt to quantify nanoparticle dose enhancement. In our benchmark test, dose-rate constant, radial dose function, and two-dimensional anisotropy function calculated with our model were within 2% of those reported previously. Using our simulation model we found that the radiation dose was enhanced up to 60% with 1013 gold nanospheres per cm3 (9.6% by weight) in a water phantom selectively around the nanospheres. The comparison study indicated that our model more accurately calculated the dose enhancement effect and that previous methodologies overestimated the dose enhancement up to 16%. Monte Carlo calculations demonstrate that biologically-relevant radiation dose enhancement can be achieved with the use of gold nanospheres. Selective tumor labeling with gold nanospheres may be a strategy for clinically enhancing radiation effects.









Similar content being viewed by others
References
S. Agostinelli, J. Allison, K. Amako et al., Geant4—a simulation toolkit. Nucl. Instrum. Methods Phys. Res. A 506, 250–303 (2003). doi:10.1016/S0168-9002(03)01368-8
J. Allison, K. Amako, J. Apostolakis et al., Geant4 developments and applications. IEEE Trans. Nucl. Sci. 53, 270–278 (2006). doi:10.1109/TNS.2006.869826
A. Angelopoulos, P. Baras, L. Sakelliou et al., Monte Carlo dosimetry of a new Ir-192 high dose rate brachytherapy source. Med. Phys. 27, 2521–2527 (2000). doi:10.1118/1.1315316
F. Ballester, C. Hernández, J. Pèrez-Calatayud et al., Monte Carlo calculation of dose rate distributions around Ir-192 wires. Med. Phys 24, 1221–1228 (1997). doi:10.1118/1.598142
F. Ballester, J. Pèrez-Calatayud, V. Puchades et al., Monte Carlo dosimetry of Buchler high dose rate Ir-192 source. Med. Phys 28, 2586–2591 (2001). doi:10.1118/1.1420398
K. Bullis, Remotely activated nanoparticles destroy cancer. Technology Review 2007. http://www.technologyreview.com/Nanotech/17956/. Published January 2, 2007. Accessed November 25, 2007
S.H. Cho, Estimation of tumor dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys. Med. Biol 50, N-163–N-173 (2005)
S.J. Douglas, S.S. Davis, L. Illum, Nanoparticles in drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 3, 133–161 (1987)
A.M. Gobin, M.H. Lee, N.J. Halas et al., Nano Lett 7, 1929–1934 (2007). doi:10.1021/nl070610y
D. Granero, J. Pèrez-Calatayud, F. Ballester, Monte Carlo calculation of the TG-43 dosimetric parameters of a new BEBIG Ir-192 HDR source. Radiother. Oncol 76, 79–85 (2005). doi:10.1016/j.radonc.2005.06.016
J.F. Hainfeld, D.N. Slatkin, H.M. Smilowitz, The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 49, 309-N–315 (2004)
J.F. Hainfeld, D.N. Slatkin, T.M. Focella et al., Gold nanoparticles: a new X-ray contrast agent. Br. J. Radiol. 79, 248–253 (2006). doi:10.1259/bjr/13169882
D.M. Herold, I.J. Das, C.C. Stobbe et al., Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol 76, 1357–1364 (2000). doi:10.1080/09553000050151637
M. Hu, J.Y. Chen, Z.Y. Li et al., Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev 35, 1084–1094 (2006). doi:10.1039/b517615h
H.E. Johns, J.W. Hunt, S.O. Fedoruk, Surface back-scatter in the 100 kV to 400 kV range. Br. J. Radiol. 27, 443–448 (1954)
K.L. Kelly, E. Coronado, L.L. Zhao et al., The optical properties of metal nanoparticles: The influences of size, shape, and dielectric environment. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys 107, 668–677 (2003)
C. Loo, A. Lowery, N. Halas, J. West, R. Drezek, Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 5(4)), 709–711 (2005). doi:10.1021/nl050127s
R. Nath, L.L. Anderson, G. Luxton et al., Dosimetry of interstitial brachytherapy source: recommendations of AAPM radiation therapy committee task group No. 43. Med. Phys 22, 209–234 (1995). doi:10.1118/1.597458
C. Noguez, Surface plasmons on metal nanoparticles: The influence of shape and physical environment. J. Phys. Chem. C. Nanomaterials Interfaces 111, 3806–3819 (2007)
NuDat 2.4. National Nuclear Data Center. Nuclear data from NuDat, a web-based database maintained by the National Nuclear Data Center. Upton, NY, USA: Brookhaven National Laboratory; http://www.nndc.bnl.gov/nudat2. Accessed November 25, 2007
J. Panyam, V. Labhasetwar, Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev 55, 329–347 (2003). doi:10.1016/S0169-409X(02)00228-4
Physics Laboratory, National Institute of Standards and Technology., Stopping-power and range tables for electrons. http://physics.nist.gov/PhysRefData/Star/Text/ESTAR.html . Accessed November 25, 2007
M.J. Rivard, B.M. Coursey, L.A. Dewerd et al., Update of AAPM task group No. 43 report: a revised AAPM protocol for brachytherapy dose calculations. Med. Phys. 31, 633–674 (2004). doi:10.1118/1.1646040
J.L. Robar, Generation and modeling of megavoltage photon beams for contrast-enhanced radiation therapy. Phys. Med. Biol 51, 5487–5504 (2006). doi:10.1088/0031-9155/51/21/007
J.L. Robar, S.A. Riccio, M.A. Martin, Tumor dose enhancement using modified megavoltage photon beams and contrast media. Phys. Med. Biol 47, 2433–2449 (2002). doi:10.1088/0031-9155/47/14/305
J.H. Sakamoto, B.R. Smith, B. Xie et al., The molecular analysis of breast cancer utilized targeted nanoparticle based ultrasound contrast agents. Technol. Cancer Res. Treat. 4, 627–636 (2005)
G. Schmid, Nanoparticles: from theory to application (Wiley-Vch Verlag GmbH & Co. KGaA; Weinheim, Germany, 2004)
D.C. Sullivan, M. Ferrari, Nanotechnology and tumor imaging: seizing an opportunity. Mol. Imaging 3, 364–369 (2004). doi:10.1162/1535350042973526
F. Verhaegen, B. Reniers, E. Deblois et al., Dosimetric and microdosimetric study of contrast-enhanced radiotherapy with kilovolt X-rays. Phys. Med. Biol 50, 3555–3569 (2005). doi:10.1088/0031-9155/50/15/005
J.F. Williamson, Monte Carlo evaluation of kerma at a point for photon transport problems. Med. Phys. 14, 567–576 (1987). doi:10.1118/1.596069
X-5 Monte Carlo Team, MCNP-A General Monte Carlo N-Particle Transport Code, Version 5. LA-UR-03-1987. Los Alamos National Laboratory: Los Alamos, NM, 2004
Conflict of interest
The authors are unaware of any actual or potential conflicts of interest that may exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was partially funded by a grant from the U. S. Department of Defense Breast Cancer Research Program (W81XWH-06-1-0672) and by the institutional core grant (CA 16672). The authors also wish to acknowledge the Department of Scientific Publications at The University of Texas M. D. Anderson Cancer Center for its editorial assistance during the preparation of this article.
Appendix
Appendix
Radiation interaction coefficients for gold nanosphere solution σ gold-nano and gold-water mixture σ gold-water
If the total cross section of each target molecule is represented by σ, the cross section of each water molecule is σ H2O and the cross section of each gold atom is σ Au , then the total interaction probability of a photon with each phantom molecule is proportional to σ. However, σ differs between the gold-water mixture and the gold nanosphere solution. In the gold-water mixture model, if it has a gold concentration of 9.7% by weight,
Where N A = 6.022 × 1023 mol−1 is the Avogadro constant.
In the gold nanosphere solution model, the cross section was calculated independently, that is
If we assume that the total number of water molecules is N1 and the total number of gold atoms is N2, then the total number of phantom molecules is N = (N1 + N2). In the gold-water mixture model, the total cross section was calculated as
In the gold nanoparticle solution model, the total cross section was calculated as
Where x% and (100-x)% are the percentages of water molecules and gold atoms in the solution, which will depend on the distribution of gold nanoparticles in the solution. It is obvious that σ gold-water is differs from σ gold-nano , which accounts for the different dose enhancement estimations generated by the two models. The gold nanoparticle geometry model we created in our study simulated the gold nanoparticle solution more closely than gold-water mixture model used in the previous studies.
Rights and permissions
About this article
Cite this article
Zhang, S.X., Gao, J., Buchholz, T.A. et al. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a monte carlo simulation study. Biomed Microdevices 11, 925–933 (2009). https://doi.org/10.1007/s10544-009-9309-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-009-9309-5