Abstract
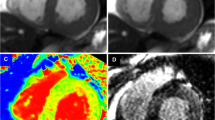
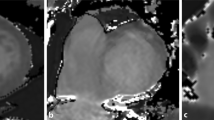
To test feasibility of myocardial T1 mapping of the right ventricle (RV) at systole when myocardium is more compact and to determine the most appropriate imaging plane. 20 healthy volunteers (11 men; 33 ± 8 years) were imaged on a 1.5T scanner (MAGNETOM Avanto, Siemens AG, Erlangen, Germany). A modified look-locker inversion-recovery sequence was acquired at mid-ventricular short axis (SAX), as horizontal long-axis view and as transversal view at systole (mean trigger time 363 ± 37 ms). Myocardial T1 time of the left-ventricular and RV myocardium was measured within a region of interest (ROI) on generated T1-maps. The most appropriate imaging plane for the RV was determined by the ability to draw a ROI including the largest amount of myocardium without including adjacent tissue or blood. At systole, when myocardium is thicker, measurements of the RV myocardium were feasible in 18/20 subjects. Average size of the ROI was 0.42 ± 0.28 cm2. In 10/18 subjects, short axis was the most appropriate imaging plane to obtain measurements (p = 0.034). Average T1 time of the RV myocardium was 1,016 ± 61 ms, and average T1 of the left-ventricular (LV) was 956 ± 25 ms (p < 0.001). T1 mapping of the RV myocardium is feasible during systole in the majority of healthy subjects but with a small ROI only. SAX plane was the optimal imaging plane in the majority of subjects. Native myocardial T1 time of the RV is significantly longer compared to the LV, which might be explained by the naturally higher collagen content of the RV.





Similar content being viewed by others
References
Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ (2008) Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 52(19):1574–1580. doi:10.1016/j.jacc.2008.06.049
Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J (2007) Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 26(4):1081–1086. doi:10.1002/jmri.21119
Messroghli D, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, Klein C, Berger F, Kuehne T (2011) Assessment of diffuse myocardial fibrosis in rats using small animal look-locker inversion recovery (SALLI) T1 mapping. Circ Cardiovasc Imaging. doi:10.1161/CIRCIMAGING.111.966796
Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, Cirino AL, Lakdawala NK, Orav EJ, Gonzalez A, Lopez B, Diez J, Jerosch-Herold M, Kwong RY (2013) T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. doi:10.1161/CIRCIMAGING.112.000333
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E (2013) Native t1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6(4):475–484. doi:10.1016/j.jcmg.2012.08.019
Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC (2010) Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 122(2):138–144. doi:10.1161/CIRCULATIONAHA.109.930636
Jellis C, Martin J, Narula J, Marwick TH (2010) Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol 56(2):89–97. doi:10.1016/j.jacc.2010.02.047
Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ (2012) Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T1 mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging 5(1):51–59. doi:10.1161/CIRCIMAGING.111.965608
Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M (2010) Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging 3(6):727–734. doi:10.1161/CIRCIMAGING.108.842096
McCann GP, Gan CT, Beek AM, Niessen HW, Vonk Noordegraaf A, van Rossum AC (2007) Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. AJR Am J Roentgenol 188(2):349–355. doi:10.2214/AJR.05.1259
Basso C, Corrado D, Marcus FI, Nava A, Thiene G (2009) Arrhythmogenic right ventricular cardiomyopathy. Lancet 373(9671):1289–1300. doi:10.1016/S0140-6736(09)60256-7
Plymen CM, Sado DM, Taylor AM, Bolger AP, Lambiase PD, Hughes M, Moon JC (2013) Diffuse myocardial fibrosis in the systemic right ventricle of patients late after mustard or senning surgery: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. doi:10.1093/ehjci/jet014
Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA (2012) T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson 14:27. doi:10.1186/1532-429X-14-27
Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JA, Bluemke DA, Kawut SM (2012) Obesity and right ventricular structure and function: the MESA-right ventricle study. Chest 141(2):388–395. doi:10.1378/chest.11-0172
Oken DE, Boucek RJ (1957) Quantitation of collagen in human myocardium. Circ Res 5(4):357–361
Weber KT (1989) Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13(7):1637–1652. doi:1097(89)90360-4
Piechnik SK, Ferreira VM, Lewandowski AJ, Ntusi NA, Banerjee R, Holloway C, Hofman MB, Sado DM, Maestrini V, White SK, Lazdam M, Karamitsos T, Moon JC, Neubauer S, Leeson P, Robson MD (2013) Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson 15:13. doi:10.1186/1532-429X-15-13
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawel-Boehm, N., Dellas Buser, T., Greiser, A. et al. In-vivo assessment of normal T1 values of the right-ventricular myocardium by cardiac MRI. Int J Cardiovasc Imaging 30, 323–328 (2014). https://doi.org/10.1007/s10554-013-0326-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-013-0326-3