Abstract
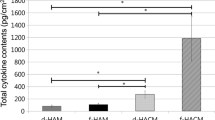
The aim of this work is to quantify the total protein and growth factors content in a tissue-suspension obtained from processed human amniotic membrane (hAM). hAM was collected, frozen, freeze dried, powdered and sterilized by γ-irradiation. At each step of the process, samples were characterized for the total protein amounts by a Bradford protein assay and for the growth factor concentrations by ELISA test of the tissue suspensions. Frozen-hAM samples show higher release of total proteins and specific growth factors in the tissue suspension in comparison with freeze-dried hAM. We observed that even if the protein extraction is hindered once the tissue is dried, the powdering process allows a greater release in the tissue suspension of total proteins and growth factors after tissue re-solubilization in comparison with only the freeze-drying process (+91 ± 13% for EGF, +16 ± 4% for HGF, +11 ± 5% for FGF, +16 ± 9% for TGF-β1), and a greater release of EGF (85 ± 10%) in comparison with only the freezing process, because proteins become much readily solubilized in the solution. According with these results, we describe a protocol to obtain a new sterile biological product from hAM tissue, with well-known effects of thermal, mechanical and physical processes on the total protein and grow factors contents.




Similar content being viewed by others
References
Azuara-Blanco A, Pillai CT, Dua HS (1999) Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol 83:399–402
Batmanov IE, Egorova KS, Kolesnikova LN (1990) Use of fresh amnion in the treatment of corneal diseases. Vestn oftalmol 106:17–19
Bonci P, Bonci P, Lia A (2005) Suspension made with amniotic membrane: clinical trial. Eur J Ophthalmol 15:441–445
Chen HJ, Pires RT, Tseng SC (2000) Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol 84:826–833
Dua HS, Gomes JAP, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv Ophthalmol 49:51–77
Hoppenreijs VPT, Pels E, Vrensen GFJM, Treffers WF (1996) Corneal endothelium and growth factors. Surv Ophthalmol 41:155–164
Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S (2000) Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 19:113–129
Jiang A, Li C, Gao Y, Zhang M, Hu J, Kuang W, Hao S, Yang W, Xu C, Gao G, Wang Z, Liu Z (2006) In vivo and in vitro inhibitory of amniotic extraction on neovascularization. Cornea 25(10 Suppl 1):S36–S40
Kim JC, Tseng SC (1995) Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 14:473–484
Koizumi NJ, Inatomi TJ, Sotozono CJ (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20:173–177
Kruse FE, Cursiefen C (2008) Surgery of the cornea: corneal, limbal stem cell and amniotic membrane transplantation. Dev Ophthalmol 41:159–170
Lee SH, Tseng SC (1997) Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 123:303–312
Lee SB, Li DQ, Tan DT (2000) Suppression of TGF-beta signalling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res 20:325–334
Meller D, Pires RT, Tseng SC (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol 86(4):463–471
Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, Sotozono C, Nakamura T, Shimizu Y, Kinoshita S (2004) Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci 45:93–99
Nakamura T, Inatomi T, Sekiyama E, Ang LPK, Yokoi N, Kinoshita S (2006) Novel clinical application of sterilized, freeze-dried amniotic membrane to treat patients with pterigium. Acta Ophthalmol Scand 84:401–405
Paridaens D, Beekhuis H, van Den Bosch W (2001) Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol 85:658–661
Pires RT, Tseng SC, Prabhasawat P (1999) Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol 117:1291–1297
Prabhasawat P, Barton K, Burkett G, Tseng SC (1997) Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 104:974–985
Robson MC, Krizek TJ (1973) The effect of human amniotic membranes on the bacteria population of infected rat burns. Ann Surg 177:144–149
Rodriguez-Ares MT, Tourino R, Capeans C, Sanchez-Salorio M (1999) Repair of scleral perforation with preserved scleral and amniotic membrane in Marfan’s syndrome. Ophthalmic Surg Lasers 30:485–487
Rodriguez-Ares MT, Lopez-Valladares JM, Tourino R, Vieites B, Gude F, Silva MT, Couceiro J (2009) Effects of lyophilization on human amniotic membrane. Acta Ophthalmol 87(issue 4):396–403. doi:10.1111/j.1755-3768.2008.01261.x
Sekiyama E, Nakamura T, Kurihara E, Cooper LJ, Fullwood NJ, Takaoka M, Hamuro J, Kinoshita S (2007) Novel sutureless transplantation of bioadhesive-coated, freeze-dried amniotic membrane for ocular surface reconstruction. Invest Ophthalmol Vis Sci 48:1528–1534
Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z, Ma JX (2004) Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci 45(6):1758–1762
Sorsby A, Symmons HM (1946) Amniotic membrane grafts in caustic burns of the eye (burns of second degree). Br J Ophthalmol 30:337–345
Sorsby A, Haythorne J, Reed H (1947) Further experience with amniotic membrane grafts in caustic burns of the eye. Br J Ophthalmol 31:409–418. doi:10.1136/bjo.31.7.409
Subrahmanyam M (1995) Amniotic membrane as a cover for microskin grafts. Br J Plast Surg 48(7):477–478
Tseng SC, Meller D, Anderson DF, Touhami A, Pires RT, Grüterich M, Solomon A, Espana E, Sandoval H, Ti SE, Goto E (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. Adv Exp Med Biol 506(Pt B):1323–1334
Acknowledgments
The authors thank Manuela Voltattorni, (MSc, PhD, Interdepartmental Centre of Biotechnological Research (CIRB) of University of Bologna) for assistance during the ELISA procedure and for the precious suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russo, A., Bonci, P. & Bonci, P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank 13, 353–361 (2012). https://doi.org/10.1007/s10561-011-9261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-011-9261-5