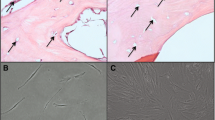
Abstract
Mesenchymal stem cells (MSCs) have been designated as the most reliable cells in clinics to treat osteo-diseases because of their versatile nature. MSCs, isolated from long bone (Lb-MSCs) are rarely reported and named as RIA-MSCs because of the reamer–irrigator–aspirator (RIA) device. The potential of these cells in the treatment of non-union bone fractures made them the ideal candidates to be studied for clinical practices. In this work, effect of cryopreservation on the proliferation and differentiation capabilities of long bone MSCs (Lb-MSCs) has been studied. For this purpose, Lb-MSCs were isolated via RIA device and characterized using flow cytometry and differentiation assays. Cells were cryopreserved for 3, 6 and 12 months and thereafter were characterized using differentiation assays and genetic markers specific for osteogenic, chondrogenic, and adipogenic potential quantitatively by qRT-PCR. Lb-MSCs were found expressing MSC characteristic markers defining their identity. The population doubling time (PDT) was about 2.5 ± 0.5 days and colonies appeared after 7–10 days. Differentiation potential and gene expression of 3, 6 and 12 months cryopreserved Lb-MSCs were unaltered. The results show that cryopreservation did not have an effect on the differentiation potential of human Lb-MSCs. Therefore, our work offers Lb-MSCs as clinically cells for treating osteo-diseases.






Similar content being viewed by others
References
Apel A et al (2009) Suitability of human mesenchymal stem cells for gene therapy depends on the expansion medium. Exp Cell Res 315(3):498–507
Baksh D, Yao R, Tuan RS (2007) Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25(6):1384–1392
Belthur MV et al (2008) Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res 466(12):2973–2980
Bianco P, Robey PG (2000) Marrow stromal stem cells. J Clin Investig 105(12):1663
Brighton CT, Hunt RM (1991) Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg 73(6):832–847
Bruder SP, Caplan AI (2000) Bone regeneration through cellular engineering. Princ Tissue Eng 2:683–696
Clarke D, Frisen J (2001) Differentiation potential of adult stem cells. Curr Opin Genet Dev 11(5):575–580
Cox G et al (2011) The use of the reamer–irrigator–aspirator to harvest mesenchymal stem cells. J Bone Joint Surg Br 93(4):517–524
Dell’Accio F et al (2003) Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res 21(1):123–131
Ding J et al (2009) TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci 84(15):499–504
Fayaz HC et al (2011) The role of stem cells in fracture healing and nonunion. Int Orthop 35(11):1587–1597
Giannoudis P, Tzioupis C, Green J (2009) Surgical techniques: how I do it? the reamer/irrigator/aspirator (RIA) system. Injury 40(11):1231–1236
Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5(5):362–369
Goh BC et al (2007) Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med 1(4):322–324
Haynesworth S et al (1992) Characterization of cells with osteogenic potential from human marrow. Bone 13(1):81–88
Henrich D et al (2010) RIA reamings and hip aspirate: a comparative evaluation of osteoprogenitor and endothelial progenitor cells. Injury 41:S62–S68
Hernigou P, Beaujean F (1996) Bone marrow in patients with pseudarthrosis. A study of progenitor cells by in vitro cloning. Rev Chir Orthop Repar L’appar Mot 83(1):33–40
Hernigou P et al (2005) Percutaneous autologous bone-marrow grafting for nonunions. J Bone Joint Surg 87(7):1430–1437
Horwitz EM et al (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5(3):309–313
Huffman LK, Harris JG, Suk M (2009) Using the bi-masquelet technique and reamer–irrigator–aspirator for post-traumatic foot reconstruction. Foot Ankle Int 30(9):895–899
Jaiswal N et al (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 64(2):295–312
Jones EA et al (2002) Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 46(12):3349–3360
Kern S et al (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301
Khanal G, Garg M, Singh G (2004) A prospective randomized trial of percutaneous marrow injection in a series of closed fresh tibial fractures. Int Orthop 28(3):167–170
Kögler G et al (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200(2):123–135
Kotobuki N et al (2005) Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng 11(5–6):663–673
Krause D (2002) Plasticity of marrow-derived stem cells. Gene Ther 9(11):754–758
Kuehlfluck P et al (2015) RIA fractions contain mesenchymal stroma cells with high osteogenic potency. Injury 46:S23–S32
Lee RH et al (2004) Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 14(4–6):311–324
Li X et al (2014) Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med 34(3):695–704
Lübke C et al (2005) Growth characterization of neo porcine cartilage pellets and their use in an interactive culture model. Osteoarthr Cartil 13(6):478–487
Mamidi MK et al (2011) Co-culture of mesenchymal-like stromal cells derived from human foreskin permits long term propagation and differentiation of human embryonic stem cells. J Cell Biochem 112(5):1353–1363
Marcacci M et al (2007) Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng 13(5):947–955
Muschler GF, Boehm C, Easley K (1997) Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am 79(11):1699–1709
Naderi-Meshkin H et al (2014) Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int 38(1):72–84
Newman JT et al (2008) A new minimally invasive technique for large volume bone graft harvest for treatment of fracture nonunions. Orthopedics 31(3):257–261
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Quarto R et al (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344(5):385–386
Ramirez-Zacarias J, Castro-Munozledo F, Kuri-Harcuch W (1992) Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 97(6):493–497
Rowley SD (1992) Hematopoietic stem cell cryopreservation: a review of current techniques. J Hematother 1(3):233–250
Sergeeva N et al (2006) Study of growth parameters and potentialities of differentiation of multipotent mesenchymal stromal cells from rat bone marrow in vitro. Bull Exp Biol Med 141(4):530–535
Sidney LE, Kirkham GR, Buttery LD (2013) Comparison of osteogenic differentiation of embryonic stem cells and primary osteoblasts revealed by responses to IL-1β, TNF-α, and IFN-γ. Stem Cells Dev 23(6):605–617
Stafford PR, Norris B (2007) Reamer–irrigator–aspirator as a bone graft harvester. Tech Foot Ankle Surg 6(2):100–107
Stolzing A, Colley H, Scutt A (2011) Effect of age and diabetes on the response of mesenchymal progenitor cells to fibrin matrices. Int J Biomater 2011:378034
Thirumala S, Goebel WS, Woods EJ (2009) Clinical grade adult stem cell banking. Organogenesis 5(3):143–154
Toosi S, Vahednia E, Behravan J (2016a) Ream content a stem cell source for bone defects. Stem Cells Transl Investig 3:e1380
Toosi S et al (2016b) Comparative characteristics of mesenchymal stem cells derived from reamer–irrigator–aspirator, iliac crest bone marrow, and adipose tissue. Cell Mol Biol 62(10):68
Ulloa-Montoya F, Verfaillie CM, Hu W-S (2005) Culture systems for pluripotent stem cells. J Biosci Bioeng 100(1):12–27
Woods EJ et al (2009) Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 59(2):150–157
Wulf GG, Jackson KA, Goodell MA (2001) Somatic stem cell plasticity: current evidence and emerging concepts. Exp Hematol 29(12):1361–1370
Yamaguchi M et al (2001) Serum-free coculture system for ex vivo expansion of human cord blood primitive progenitors and SCID mouse-reconstituting cells using human bone marrow primary stromal cells. Exp Hematol 29(2):174–182
Acknowledgements
The authors are indebted to the Research Council of the Mashhad University of Medical Sciences, Iran, for its approval and financial support of this research. This study was extracted from the Ph.D. thesis of Shirin Toosi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toosi, S., Naderi-Meshkin, H., Kalalinia, F. et al. Long bone mesenchymal stem cells (Lb-MSCs): clinically reliable cells for osteo-diseases. Cell Tissue Bank 18, 489–500 (2017). https://doi.org/10.1007/s10561-017-9652-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9652-3