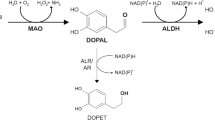
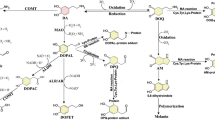
1. Parkinson’s disease (PD) is considered to be an aging-related neurodegeneration of catecholamine (CA) systems [typically A9 dopamine (DA) neurons in the substantia nigra and A6 noradrenaline (NA) neurons in the locus coeruleus]. The main symptom is movement disorder caused by a DA deficiency at the nerve terminals of fibers that project from the substantia nigra to the striatum. Most PD is sporadic (sPD) without any hereditary history. sPD is speculated to be caused by some exogenous or endogenous substances that are neurotoxic toward CA neurons, which toxicity leads to mitochondrial dysfunction and subsequent oxidative stress resulting in the programmed cell death (apoptosis or autophagy) of DA neurons.
2. Recent studies on the causative genes of rare familial PD (fPD) cases, such as alpha–synuclein and parkin, suggest that dysfunction of the ubiquitin–proteasome system (UPS) and the resultant accumulation of misfolded proteins and endoplasmic reticulum stress may cause the death of DA neurons.
3. Activated microglia, which accompany an inflammatory process, are present in the nigro-striatum of the PD brain; and they produce protective or toxic substances, such as cytokines, neurotrophins, and reactive oxygen or nitrogen species. These activated microglia may be neuroprotective at first in the initial stage, and later may become neurotoxic owing to toxic change to promote the progression toward the death of CA neurons.
4. All of these accumulating evidences on sPD and fPD points to a hypothesis that multiple primary causes of PD may be ultimately linked to a final common signal-transduction pathway leading to programmed cell death, i.e., apoptosis or autophagy, of the CA neurons.
Similar content being viewed by others
REFERENCES
Abott, A. (2005). While you are sleeping. Nature (News feature)437:1220–1222.
Anglade, P., Vyas, S., Javoy-Agid, F., Ilerreto, M. T., Michel, P. P., Marquez, J., Pouatt-Prigent, A., Ruberg, M., Hirsch, C., and Agid, Y. (1997). Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 12:25–31.
Axelrod, J., Weil-Malherbe, H., and Tomchick, R. (1959). The physiological distribution of 3H-epinephrine and its metabolite epinephrine. J. Pharm. Exp. Therap. 127:251–256.
Axelrod, J. (1957). O-Methylation of epinephrine and other catecholamines in vitro and in vivo. Science: 126:400–401.
Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., and Greenmyre, J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurobiol. 3:1301–1306.
Blum-Degan, D., Mueller, T., Kuhn, W., Gerlach, M., Przuntek, H., and Riederer, P. (1995). Interleukin 1-beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease. Neurosci. Lett. 202:17–20.
Boka, G., Anglade, P., Wallach, D., Javoy-Azid, F., Agid, Y., and Hirsch, E. C. (1994). Immunocytochemical analysis of tumor necrosis factor and its receptor in Parkinson’s disease. Neurosci. Lett 172:151– 154.
Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O. J., Breedveld, G. J., Krieger, E., Dekker, M. C. J., Squitieri, F., Ibanez, P., Joosse, M., van Dongen, J. W., Vanacore, N., van Swieten, J. C., Brice, A., Meco, G., van Duijn, C. M., Oostra, B. A., and Heutink, P. (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299:256–259.
Bonifati, V., Oostra, B. A., and Heutink, P. (2004). Unraveling the pathogenesis of Parkinson’s disease: the contribution of monogenic forms. Cell. Mol. Life Sci. 61:1729–1750.
Braak, H., DelTredici, K., Rub, U., deVos, R. A. I., Steur, E. N. H. J., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24:197–212.
Carlsson, A. (1959). The occurrence, distribution and physiological role of dopamine in the nervous system. `Pharmacol. Rev. 11:490–493.
Chandra, S., Gallardo, G., Fernandez-Chacon, R., Schlueter, O. M., and Suedhof, T. C. (2005). Alpha-synuclein cooperates with CSP alpha in preventing neurodegeneration. Cell 123:383–396.
Chen, L., and Feany, M. B. (2005). Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson’s disease. Nat. Neurosci. 8:657–663.
Chiba-Falek, O., and Nussbaum, R. L. (2003). Regulation of alpha-synuclein expression: Implication for Parkinson’s disease. Cold Spring Harbor Sym. Quantit. Biol. LXVIII:409–415.
Chung, K. K., Dawson, V. L., and Dawson, T. M. (2001). The role of the ubiquitin-proteasomal pathway in Parkinson’s disease and other neurodegenerative disorders. Trends Neurosci. 24(Suppl. 11):S7–S14.
Chung, K. K., Thomas, B., Li, X., Pletnikova, O., Troncosa, J. C., Marsh, L., Dawson, V. L., and Dawson, T. M. (2004). S-Nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science 304:1328–1331.
Collins, M. A., and Neafsey, E. J. (2000). Beta-carboline analogues of MPP+ as environmental neurotoxins. In Storch, A., and Collins, M. A. (eds.), Neurotoxic Factors in Parkinson’s Disease and Related Disorders, Kluwer Academic Publishing/Plenum, New York, pp. 115–130.
Conway, K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Williamson, R. E., and Lansbury, P. T., Jr. (2000). Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early onset Parkinson’s disease: Implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 97:571–576.
Cookson, M. R. (2005). The biochemistry of Parkinson’s disease. Ann. Rev. Biochem. 74:29–74.
Dawer, W., and Przedborski, S. (2003) Parkinson’s disease: Mechanisms and models, Neuron 39:889– 909.
Davis, G. C. B., Williams, A. C., Markey, S. P., Ebert, M. H., Caine, E. D., Reichert, C. M., and Kopin, I. J. (1979). Chronic parkinsonism secondary to intravenous injection of meperidine analogus. Psychiatry Res. 1:249–254.
Di Fonzo, A., Rohe, C. F., Ferreira, J., Chien, H. F., Vacca, L., Stocchi, F., Guedes, L., Fabrizio, E., Manfredi, M., Vanacore, N., Goldwurm, S., Breedveld, G., Sampaio, C., Meco, G., Barbosa, E., Oostra, B. A., and Bonifati, V. Italian Parkinson Genetics Network (2005). A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet 365:412–415.
Ehringer, H., and Hornykiewicz, O. (1960). Verteilung von Noradrenalin und Dopamin (3-Hydroxytyramin) im Gehirn des Menschen und ihr Verhalten bei Erkarankungen des extrapyramidalen Systems. Klin. Wschr. 38:1236–1239.
Eisenhofer, G., Lamensdorf, I., Kirk, K. L., Kawamura, M., and Sato, S. (2002). Oxidative deamination of monoamines and biogenic aldehydes in neurodegenetration. In Creveling, C. R. (ed.), Role of Catechol Quinone Species in Cellular Toxicity, F.P. Graham Publishing, Johnson City, pp. 147–167.
Feany, M. B. (2004). New genetic insights into Parkinson’s disease. New Engl. J. Med. 351:1937–1940.
Feany, M. B., and Bender, W. W. (2000). A Drosophila model of Parkinson’s disease. Nature 404:394–398.
Foley, P., and Riederer, P. (1999). Pathogenesis and preclinical course of Parkinson’s disease. J. Neural. Transm. Suppl. 56:31–74.
Forman, M. S., Trojanowski, J. Q., and Lee, V. M.-Y. (2004). Neurodegenerative diseases: A decade of discoveries paves the way for therapeutic breakthroughs. Nature Med. 10:1055–1063.
Fornai, F., Schlueter, O. M., Lenzi, P., Gesi, M., Ruffoli, R., Ferrucci, M., Lazzeri, G., Busceti, C. L., Pontarelli, F., Battaglia, G., Pellegrini, A., Nicoletti, F., Ruggieri, S., Paparelli, A., and Suedhof, T. C. (2005). Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc. Natl. Acad. Sci. USA 102:3413–3418.
Fukuda, T. (1994). 1-Methyl-1,2,3,4-tetrahydroisoquinoline does dependently reduces the number of tyrosine hydroxylase-immunoactive cells in the substantia nigra and locus ceruleus of C57BL/6J mice. Brain Res. 639:325–328.
Gerlach, M., Ben-Shachar, D., Riederer, P., and Youdim, M. B. H. (1994). Altered brain metabolism of iron as a cause of neurodegenerative diseases? J. Neurochem. 63:793–807.
Goedert, M. (2001). Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2:492–501.
Goldstein, D. S., Eldadah, B. A., Holmes, C., Pechnik, S., Moak, J., Saleem, A., and Sharabi, Y. (2005). Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotention: Independence from Levodopa treatment. Hypertension 46:1333–1339.
Grandhi, S., and Wood, N. W. (2005). Molecular pathogenesis of Parkinson’s disease. Human Mol. Genet. 14:2749–2755.
Hartmann, A., Hunot, S., Michel, P. P., Muriel, M. P., Vyas, S., Faucheux, B. A., Mouatt-Prignet, A., Turmel, H., Srinivasan, A., Ruberg, M., Evan, G. I., Agid, Y., and Hirsch, E. C. (2000). Caspase-3: A vulnerable factor and a final effector in the apoptotic cell death of dopaminergic neurons in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 97:2875–2880.
Hasbani, D. M., Perez, F. A., Palmiter, R. D., and O’Malley, K. L. (2005). Dopamine depletion does not protect against acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in vivo. J. Neurosci. 25:9428–9433.
Hayley, S. (2005). Multiple mechanisms of cytokine activation in neurodegenerative and psychiatric states: Neurochemical and molecular substrates. Curr. Pharmac Design 11:947–962.
Hirsch, E. C., Hunot, S., Faucheux, B. A., Agid, Y., Mizuno, Y., Mochizuki, H., Tatton, W. G., Tatton, N., and Olanow, W. C. (1999). Dopaminergic neurons degenerate by apoptosis in Parkinson’s disease. Mov. Disord. 14:383–385.
Hoffmann, G. F., Assmann, B., Braeutigam, C., Dionisi-Vici, C., Haeussler, M., and deKlerk, J. B. C., Neumann, M., Steenbergen-Spanjers, G. C. H., Strassburg, M.-H., and Wevers, R. A. (2003). Tyrosine hydroxylase deficiency causes progressive encephalopathy and dopa-non-responsive dystonia. Ann. Neurol. 54(Suppl. 6):S56–S65.
Honbou, K., Suzuki, N. N., Horiuchi, M., Niki, T., Taira, T., Ariga, H., and Inagaki, F. (2003). The crystal structure of DJ-1, a protein related to male fertility and`Parkinson’s disease. J. Biol. Chem. 278:31380–31384.
Ichinose, H., Ohye, T., Takahashi, E., Seki, N., Hori, T., Segawa, M., Nomura, Y., Endo, K., Tanaka, H., Tsuji, S., Fujita, K., and Nagatsu, T. (1994). Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydolase I gene. Nature Genet. 8:236–242.
Ichinose, H., Ohye, T., Matsuda, Y., Hori, T., Blau, N., Burlina, A., Rouse, B., Matalon, R., Fujita, K., and Nagatsu, T. (1995). Characterization of mouse and human GTP cyclohydrolase I genes. Mutations in patients with GTP cyclohydrolase I deficiency. J. Biol. Chem. 270:10062–10071.
Ichinose, H., Suzuki, T., Inagaki, H., Ohye, T., and Nagatsu, T. (1999). Molecular genetics of dopa-responsive dystonia. Biol. Chem. 380:1355–1364.
Ichinose, H., Ohye, T., Suzuki, T., Sumi-Ichinose, C., Nomura, T., Hagino, Y., and Nagatsu, T. (1999). Molecular cloning of the human Nurr1 gene: Characterization of the human gene and cDNA. Gene 230:233–239.
Ikemoto, K., Nagatsu, I., Ito, S., King, R., Nishimura, A., and Nagatsu, T. (1998). Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 253:198–200.
Imai, Y., Soda, M., Inoue, H., Hattori, N., Mizuno, Y., and Takahashi, R. (2001). An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of parkin. Cell 105:891–902.
Imamura, K., Hishikawa, N., Sawada, M., Nagatsu, T., Yosida, M., and Hashizume, Y. (2003). Distribution of major histocompatibility complex II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 106:518–526.
Imamura, K., Hishikawa, N., Ono, K., Suzuki, H., Sawasa, M., Nagatsu, T., Yoshida, M., and Hashizume, Y. (2005). Cytokine production of activated microglia and decrease on neurotrophic factors of neurons in the hippocampus of Lewy body disease brain. Acta Neuropathol. 109:141–150.
Ischiropoulos, H., and Beckman, J. S. (2003). Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Invest. 111:163–169.
Iwawaki, T., Kohno, K., and Kobayashi, K. (2000). Identification of a potential Nurr1 response element that activates the tyrosine hydroxylase gene promoter in cultured cells. Biochem. Biophys. Res. Cpmmun. 274:590–595.
Kajita, M., Niwa, T., and Nagatsu, T. (2002). Tetrahydroisoquinolines (TIQ) and neurodegeneration. In Creveling, C. R. (ed.), Role of Quinone Species in Cellular Toxicity, F. P. Graham Publishing, Johnson City, TN, pp. 169–190.
Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y., and Shimizu, N. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608.
Kobayashi, K., and Nagatsu, T. (2005). Molecular genetics of tyrosine 3-monooxygenase and inherited diseases. Biochem. Biophy. Res. Commun. 338:267–270.
Kosaka, K. (2000). Lewy body disease. Neuropathol. Suppl. 20:73–78.
Kostrzewa, R. M., and Jacobowitz, D. M. (1974). Pharmacological actions of 6-hydroxydopamine. Pharm. Rev. 26:199–288.
Kotake, Y., Tasaki, Y., Makino, S., Hirobe, M., and Ohta, S. (1995). 1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: A novel endogenous amine in mouse brain and parkinsonian CSF. J. Neurochem. 65:2633–2638.
Krueger, R. (2004). Genes in familial parkinsonism and their role in sporadic Parkinson’s disease. J. Neurol. 251(Supl. 6 ):VI/2–VI/6.
Kuhn, W., Mueller, Th., Grosse, H., and Rommelspacher, H. (1996). Elevated levels of harman and norharman in cerebrospinal fluid of Parkinsonian patients. J. Neural. Transm. 103:1435–1440.
Kumar, S. (1995). ICE-like proteases in apoptosis. Trends Biochem. Sci. 20:198–202.
Langston, J. W., Ballard, P., Tetrud, J. W., and Irwin, I. (1983). Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980.
LaVoie, M. J., Ostaszewski, B. L., Weihofen, A., Schlossmacher, M. G., and Selkoe, D. J. (2005). Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 11:1214–1221.
Leroy, E., Boyer, R., Auburger, G., Leube, B., Ulm, G., Mezey, E., Harta, G., Brownstein, M. J., Jonnalagada, S., Chernova, T., Dehejia, A., Lavedan, C., Gasser, T., Steinbach, P. J., Wilkinson, K. D., and Polymeropoulos, M. H. (1998). The ubiquitin pathway in Parkinson’s disease. Nature 395:451–452.
Le, W., Xu, P., Jankovic, J., Jiang, H., Appel, S. H., Smith, R. G., and Vassilatis, K. (2003). Mutations in Nr4A2 associated with familial Parkinson’s disease. Nat. Genet. 33:85–89.
Liani, E., Eyal, A., Avraham, E., Shemer, R., Szargel, R., Berg, D., Bornemann, A., Riess, O., Ross, C. A., Rott, R., and Engelender, S. (2004). Ubiquitination of synphilin-1 and alpha-synyclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson’s disease. Proc. Nat. Acad. Sci. USA 101:5500–5505.
Lozano, A. M., and Kalia, S. K. (2005). New movement in Parkinson’s. Scientific American, pp. 68–75.
MacKeigan, J. P., Murphy, L. O., and Blenis, J. (2005). Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nature Cell Biol. 7:591–600.
Martin, L. J., Pan, Y., Price, A. C., Sterling, W., Copeland, N. G., Jenkins, N. A., Price, D. L., and Lee, M. K. (2006). Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26:41–50.
Marshall, K. A., Daniel, S. E., Cairns, N., Jenner, P., and Halliwell, B. (1997). Upregulation of the anti-apoptotic protein Bcl-2 may be an early event in neurodegeneration: Studies on Parkinson’s and incidental Lewy body disease. Biochem. Biophys. Res. Commun. 240:84–87.
Matsubara, K. (2000). N-Methyl-beta-carbolinium neurotoxins in Parkinson’s disease. In Storch, A., and Collins, M. A. (eds.) Neurotoxic Factors in Parkinson’s Disease and Related Disorders, Kluwer Academic Publishing/Plenum, New York, pp. 131–143.
Matsubara, K., Kobayashi, S., Kobayashi, Y., Yamashita, K., Koide, H., Hatta, M, Iwamoto, K., Tanaka, O., and Kimura, K. (1995). Beta-carbolinium cations, endogenous MPP+ analogs in the lumbar cerebrospinal fluid of parkinsonian patients. Neurology 45:2240–2245.
Mattammal, M. B., Chung, H. D., and Strong, R. (1993). Confirmation of a dopamine metabolite in parkinsonian brain tissue by gas-chromatography-mass spectrometry. J. Chromatogr. B 614:205–212.
Mattammal, M. B., Haring, J. H., Chung, H. D., Raghu, G., and Strong, R. (1995). An endogenous dopaminergic neurotoxin: Implication for Parkinson’s disease. Neurodegeneration 4:271–281.
McGeer, P. L., Itagaki, S., Boyes, B. E., and McGeer, E. G. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s disease and Alzheimer’s disease brain. Neurology 38:1285–1291.
McGeer, P. L., and McGeer, E. G. (1995). The inflammatory response system of brain, implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Rev. 21:195–218.
McNaught, K. S., Perl, D. P., Brownell, A. L., and Olanow, C. W. (2004). Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann. Neurol. 56:149–162.
Mizuno, Y., Yoshino, H., Ikebe, S., Hattori, N., Kobayashi, T., Shimoda-Matsubayashi, S., Matsumine, H., and Kondo, T. (1998). Mitochondrial dysfunction in Parkinson’s disease. Ann. Neurol. 44(Suppl 1):S99–S109.
Mizuno, Y. (2006). Progress in familial Parkinson’s disease. In Riederer, P. (eds.), Proceedings of the 16th International Congress on Parkinson’s Disease and Related Disorders. J. Neural Transm, in press.
Mochizuki, H., Nishi, K., and Mizuno, Y. (1993). Iron-melanin complex is toxic to dopaminergic neurons in a nigrostriatal co-culture. Neurodegeneration 2:1–7.
Mogi, M., Harada, M., Riederer, P., Narabayashi, H., Fujita, K., and Nagatsu, T. (1994a). Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 165:208–210.
Mogi, M., Harada, M., Kondo, T., Riderer, P., Inagaki, H., Miura, M., and Nagatsu, T. (1994b). Interleukin 1-beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180:147–150.
Mogi, M., Harada, M., Kondo, T., Mizuno, Y., Narabayashi, H., Riederer, P., and Nagatsu, T. (1996). bcl-2 Protein is increased in the brain from parkinsonian patients. Neurosci. Lett. 215:137–139.
Mogi, M., Togari, A., Ogawa, M., Ikeguchi, K., Shizuma, N., Fan, D.-S., Nakano, I., and Nagatsu, T. (1998). Effects of repeated administration of 1-methyl-4-phenyl-1, 2, 3, 6- tetrahydropyridine (MPTP) to mice on interleukin-1beta and nerve growth factor in the striatum. Neusci. Lett. 250:25–28.
Mogi, M., and Nagatsu, T. (1999). Neurotrophins and cytokines in Parkinson’s disease. Adv. Neurol. 80:135–139.
Mogi, M., Togari, A., Kondo, T., Mizuno, Y., Komure, O., Kuno, S., Ichinose, H., and Nagatsu, T. (2000). Caspase activities and tumor necrosis factor receptor R1 level are elevated in the substantia nigra in Parkinson’s disease. J. Neural. Transm. 107:335–341.
Moser, A., and Koempf, D. (1992). Presence of methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolines, derivatives of the neurotoxin isoquinoline, in parkinsonian lumbar CSF. Life Sci. 50:1885–1891.
Nagakubo, D., Taira, T., Kitaura, H., Ikeda, M., Tamai, K., Iguchi-Ariga, S. M. M., and Ariga, H. (1997). DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 231:509–513.
Nagata, S., and Goldstein, P. (1995). The Fas death factor. Science 267:1449–1456.
Nagatsu, T. (1997). Isoquinoline neurotoxins and Parkinson’s disease. Neurosci. Res. 29:99–111.
Nagatsu, T. (2002a). Parkinson’s disease: Changes in apoptosis-related facors suggesting possible gene therapy. J. Neural Transm. 109:731–745.
Nagatsu, T. (2002b). Amine-related neurotoxins in Parkinson’s disease. Past, present, and future. Neurotoxicol Teratol 24:565–569.
Nagatsu, T., and Ichinose, H. (1999). Molecular biology of catecholamine-related enzymes in relation to Parkinson’s disease. Cell. Mol. Neurobiol. 19:57–66.
Nagatsu, T., Mogi, M., Ichinose, H., Togari, A., and Riederer, P. (1999). Cytokines in Parkinson’s disease. NeuroSci. News 2:88–90.
Nagatsu, T., Mogi, M., Ichinose, H., and Togari, H. (2000a). Cytokines in Parkinson’s disease. J. Neural. Transm. Suppl 58:143–151.
Nagatsu, T., Mogi, M., Ichinose, H., and Togari, A. (2000b). Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural Transm. Suppl. 60:277–290.
Nagatsu, T., and Sawada, M. (2005). Inflammatory process in Parkinson’s disease: Role for cytokines. Curr. Pharmac. Design 11:999–1016.
Naoi, M., Maruyama, W., Dostert, P., Hashizume, Y., Nakahara, D., Takahashi, T., and Ota, M. (1996). Dopamine-derived endogenous 1(R), 2(N)-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, N-methyl-(R)-salsolonol, induced parkinsonism in rats: Biochemical, pathological and behavioral studies. Brain Res. 709:285–295.
Niwa, T., Takeda, N., Kaneda, N., Hashizume, Y., and Nagatsu, T. (1987). Presence of tetrahydroisoquinoline and 2-methyl-tetrahydroisoquinoline in parkinsonian and normal human brains. Biochem. Biophys. Res. Commun. 144:1084–1089.
Norris, E. H., Giasson, B. I., and Lee, V. M. (2004). Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr. Top. Dev. Biol. 60:17–54.
Ohta, S., Kohno, M., Makino, Y., Tachikawa, O., and Hirobe, O. (1997). Tetrahydroisoquinoline and 1-methyl-tetrahydroisoquinoline are present in the human brain. Biomed. Res. 8:453–456.
Paisan-Ruiz, C., Jain, S., Evans, E. W., Gilks, W. P., Simon, J., van der Brug, M., de Munain, A. L., Aparicio, S., Gil, A. M., Khan, N., Johnson, J., Martinez, J. R., Nicholl, D., Carrera, I. M., Pena, A. S., de Silva, R., Lees, A., Marti-Masso, J. F., Perez-Tur, J., Wood, N. W., and Singleton, A. B. (2004). Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44:595– 600.
Panov, A., Dikalov, S., Shalbuyeva, N., Taylor, G., Sherer, T., and Greenamyre, J. T. (2005). Rotenone model of Parkinson’s disease: Multiple brain mitochondria dysfunctions after short-term systemic rotenone intoxication. J. Biol. Chem. 280:42026–42035.
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Deheijia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenrous, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoiosin, R. C., DiIorio, G., Golbe, L. I., and Nussbaum, R. L. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047.
Sacchetti, P., Mitchell, T. R., Grameman, J. G., and Bannon, M. J. (2001). Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J. Neurochem. 76:1565–1572.
Sawada, M., Imamura, K., and Nagatsu, T. (2006). Role of cytokines in inflammatory process in Parkinson’s disease. In Riederer, P. (ed.) Proceedings of the 16th International Congress on Parkinson’s disease and Related Disorders. J. Neural Transm. in press.
Schapira, A. H. V., Gu, M., Taanman, J.-W., Tabrizi, S. J., Seaton, T., Cleeter, M., and Cooper, J. M. (1998). Mitochondria in the etiology and pathogenesis of Parkinson’s disease. Ann. Neurol. 44(Suppl 1):S89–S98.
Segawa, M., Nomura, Y., and Nishiyama, N. (2003). Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease). Ann. Neurol. 54(Suppl. 6 ):S32–S45.
Selkoe, D. (2004). Cell biology of protein misfolding: The example of Alzheimer’s and Parkinson’s disease. Nat. Cell Biol 6:1054–1061.
Shen, J. (2004). Protein kinases linked to the pathogenesis of Parkinson’s disease. Neuron 44:575–577.
Shimura, H., Hattori, N., Kubo, S., Mizuno, Y., Asakawa, S., Minoshima, S., Shimizu, N., Imai, K., Chiba, T., Tanaka, K., and Suzuki, T. (2000). Familial Parkinson gene product, parkin, is a ubiquitin-protein ligase. Nature Genet 25:302–305.
Shimura, H., Schlossmacher, M. G., Hattori, N., Frosch, M. P., Trockenbacher, A., Schneider, R., Mizuno, Y., Kosik, K. S., and Selko, D. J. (2001). Ubiquitination of a new form of alpha-synuclein by parkin from human brain: Implications for Parkinson’s disease. Science 293:263–269.
Sherer, T. B., Kim, J. H., Batarbet, R., and Greenamyre, J. T. (2003). Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 179:9–16.
Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., and Nussbaum, R. (2003). Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302:841.
Snyder, S. H. (2005). Messengers of life and death. Society for Neuroscience 2005: Program No. 467.
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998). Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 95:6469–6473.
Tasaki, Y., Makino, Y., Ohta, S., and Hirobe, M. (1991). 1-Methyl-1.2.3.4-tetrahydroisoquinoline, decreased in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mouse, prevents parkinsonism-like behavior abnormalities. J. Neurochem. 57:1940–1943.
Tatton, W., Chalmers-Redman, R., and Tatton, N. (2003). Neuroprotection by deprenyl and other propargylamines: Glyceraldehyde-3-phosphate dehydrogenase rather than monoamine oxidase. J. Neural Transm. 110:509–515.
Tretter, L., Sipos, I., and Adam-Vizi, V. (2004). Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson’s disease. Neurochem. Res. 29:569–577.
Valente, E. M., Abou-Sleiman, P. M., Caputo, V., Muqit, M. M. K., Harvey, K., Gispert, S., Ali, Z., Del Turco, D., Bentivoglio, A. R., Healy, D. G., Albanese, A., Nussbaum, R., Gonzalez-Maldonado, R., Deller, T., Salvi, S., Cortelli, P., Gilks, W. P., Latchman, D. S., Harvey, R. J., Dallapiccola, B., Auburger, G., and Wood, N. W. (2004). Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160.
Vawter, M. P., Dillon-Carter, O., Tourtellotte, W. W., Carvey, P., and Freed, W. J. (1996). TGF beta1 and TGF beta2 concentrations are elevated in Parkinson’s disease in ventricular cerebrospinal fluid. Exp. Neurol. 142:313–322.
Vila, M., and Przedborski, S. (2004). Genetic clues to the pathogenesis of Parkinson’s disease. Nat. Med. S58–S62.
Vilhardt, F., Plastre, O., Sawada, M., Suzuki, K., Wiznerowicz, M., Kiyokawa, E., Trono, D., and Krause, K.-H. (2002). The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 277:42136–42143.
Warbt, S., MacDonald, M. L. E., and Abrahams, B. S. (2003). New mutations, new etiologies for Parkinson disease. Clin. Genet. 63:352–357.
Webb, J. L., Ravikumar, B., Atkins, J., Skepper, J. N., and Rubinstein, D. C. (2003). Alpha-synclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 278:25009–25013.
Wood, P. L. (2003). Microglia: Role of microglia in chronic neurodegeneration. In Wood, P. L. (ed.) Neuroinflammation. Humana Press, Totowa, NJ, pp. 3–27.
Yamada, M., Iwatsubo, T., Mizuno, Y., and Mochizuki, H. (2004). Overexpression of alpha-synuclein in rat substantia nigra and activation of caspase-9: Resemblance to pathogenetic changes in Parkinson’s disease. J. Neurochem. 91:451–461.
Yang, Y., Nishimura, I., Imai, Y., Takahashi, R., and Lu, B. (2003). Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron 37:911–924.
Youdim, M. B. H., and Riederer, P. (1997). Understanding Parkinson’s disease. Scientific American, pp. 82–89.
Zimprich, A., Biskup, S., Leitner, P., Lichtner, P., Farrer, M., Lincoln, S., Kachergus, J., Hulihan, M., Uitti, R. J., Calne, D. B., Stoessel, A. J., Pfeiffer, R. F., Patenge, N., Carbajal, I. C., Vieregge, P., Asmus, F., Mueller-Myhsok, B., Dickson, D. W., Meitinger, T., Strom, T. M., Wszolek, Z. K., and Gasser, T. (2004). Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–607.
ACKNOWLEDGMENTS
Toshi Nagatsu dedicates this paper to the late Dr. Julie Axelrod with great admiration for him for his outstanding scientific achievements as Nobel Laureate and for his extremely warm personality and humanitarian efforts. This work was supported by grants-in-aid for scientific research from the Ministry of Labor and Welfare of Japan (MS), from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MS), and from the Japan Health Sciences Foundation (MS). We are thankful to all of our collaborators, especially Drs. K. Imamura, K. Ono, H. Suzuki, Y. Hashizume, and M. Mogi and to Drs. P. Riederer, Y. Mizuno, T. Kondo, and S. Kuno for their collaboration in supplying us post mortem brain samples from their brain banks.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue dedicated to Dr. Julie Axelrod
Rights and permissions
About this article
Cite this article
Nagatsu, T., Sawada, M. Cellular and Molecular Mechanisms of Parkinson’s Disease: Neurotoxins, Causative Genes, and Inflammatory Cytokines. Cell Mol Neurobiol 26, 779–800 (2006). https://doi.org/10.1007/s10571-006-9061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-006-9061-9