Abstract
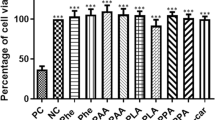
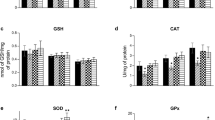
High levels of phenylalanine (Phe) are the biochemical hallmark of phenylketonuria (PKU), a neurometabolic disorder clinically characterized by severe mental retardation and other brain abnormalities, including cortical atrophy and microcephaly. Considering that the pathomechanisms leading to brain damage and particularly the marked cognitive impairment in this disease are poorly understood, in the present study we investigated the in vitro effect of Phe, at similar concentrations as to those found in brain of PKU patients, on important parameters of oxidative stress in the hippocampus and cerebral cortex of developing rats. We found that Phe induced in vitro lipid peroxidation (increase of TBA-RS values) and protein oxidative damage (sulfhydryl oxidation) in both cerebral structures. Furthermore, these effects were probably mediated by reactive oxygen species, since the lipid oxidative damage was totally prevented by the free radical scavengers α-tocopherol and melatonin, but not by L-NAME, a potent inhibitor of nitric oxide synthase. Accordingly, Phe did not induce nitric oxide synthesis, but significantly decreased the levels of reduced glutathione (GSH), the major brain antioxidant defense, in hippocampus and cerebral cortex supernatants. Phe also reduced the thiol groups of a commercial GSH solution in a cell-free medium. We also found that the major metabolites of Phe catabolism, phenylpyruvate, phenyllactate and phenylacetate also increased TBA-RS levels in cerebral cortex, but to a lesser degree. The data indicate that Phe elicits oxidative stress in the hippocampus, a structure mainly involved with learning/memory, and also in the cerebral cortex, which is severely damaged in PKU patients. It is therefore presumed that this pathomechanism may be involved at least in part in the severe cognitive deficit and in the characteristic cortical atrophy associated with dysmyelination and leukodystrophy observed in this disorder.






Similar content being viewed by others
References
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Bauman ML, Kemper TL (1982) Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta Neuropathol 58:55–63
Behl C, Moosmann B (2002a) Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med 33:182–191
Behl C, Moosmann B (2002b) Oxidative nerve cell death in Alzheimer’s disease and stroke: antioxidants as neuroprotective compounds. Biol Chem 383:521–536
Berg D, Youdim MB (2006) Role of iron in neurodegenerative disorders. Top Magn Reson Imaging 17:5–17
Bickel H, Gerrard J, Hickmans EM (1953) Influence of phenylalanine intake on phenylketonuria. Lancet 265:812–813
Bird S, Miller NJ, Collins JE, Rice-Evans CA (1995) Plasma antioxidant capacity in two cases of tyrosinemia type 1: one case treated with NTBC. J Inherit Metab Dis 18:123–126
Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF (2001) Increased oxidative damage to DNA in a transgenic mouse model of Huntington’s disease. J Neurochem 79:1246–1249
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352
Colome C, Sierra C, Vilaseca MA (2000) Congenital errors of metabolism: cause of oxidative stress? Med Clin 115:11–117
Costabeber E, Kessler A, Dutra-Filho CS, Wyse ATS, Wajner M, Wannmacher CMD (2003) Hyperfenilalaninemia reduces creatine kinase activity in the cerebral cortex of rats. Int J Dev Neurosci 21:111–116
Darling G, Mathias P, O’Regan M, Naughten E (1992) Serum selenium levels in individuals on PKU diets. J Inherit Metab Dis 15:769–773
de Oliveira Marques F, Hagen ME, Pederzolli CD (2003) Glutaric acid induces oxidative stress in brain of young rats. Brain Res 964:153–158
Embury JE, Charron CE, Martynyuk A, Zori AG, Liu B, Ali SF, Rowland NE, Laipis PJ (2007) PKU is a reversible neurodegenerative process within the nigrostriatum that begins as early as 4 weeks of age in Path(enu2) mice. Brain Res 1127:136–150
Ercal N, Aykin-Burns N, Gurer-Orhan H, McDonald JD (2002) Oxidative stress in a phenylketonuria animal model. Free Radic Biol Med 32:906–911
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388:261–266
Fighera MR, Bonini JS, de Oliveira TG, Frussa-Filho R, Dutra-Filho CS, Rubin MA, Mello CF (2003) GM1 ganglioside attenuates convulsions and thiobarbituric acid reactive substances production induced by the intraestriatal injection of methylmalonic acid. Int J Biochem Cell Biol 35:465–473
Følling A (1934) Über Ausscheidung von Phenylbrenztraubensäure in den Harn als Stoffwechseanomalie in Verbindung mit Imbezillität. Z Physiol Chem 227:169–176
Fontella FU, Pulrolnik V, Gassen E, Wannmacher CMD, Klein AB, Wajner M, Dutra-Filho CS (2000) Propionic and l-methylmalonic acids induce oxidative stress in brain of young rats. Neuroreport 11:541–544
Glushakov AV, Dennis DM, Summers C, Seubert CN, Martynyuk AE (2003) l-Phenylalanine selectively depress currents at glutamatergic excitatory synapses. J Neurosci Res 72:116–124
Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Summers C, Laipis PJ, Embury JE, Baker SP, Otero DH, Dennis DM, Seubert CN, Martynyuk AE (2005) Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain 128:300–307
Hagen MEK, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wannmacher CMD, Wyse ATS, Dutra-Filho CS (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586:344–352
Halliwell B, Gutteridge JMC (1996) Oxygen radicals and nervous system. Trends Neurosci 8:22–26
Halliwell B, Gutteridge JMC (2007) Measurement of reactive species. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 268–340
Hasselbalch S, Knudsen GM, Toft PB, Høgh P, Tedeschi E, Holm S, Videbaek C, Henriksen O, Lou HC, Paulson OB (1996) Cerebral glucose metabolism is decreased in white matter changes in patients with phenylketonuria. Pediatr Res 40:21–24
Hörster F, Schwab MA, Sauer SW, Pietz J, Hoffmann GF, Okun JG, Kölker S, Kins S (2006) Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr Res 59:544–548
Huttenlocher PR (2000) The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 159:S102–S106
Joseph B, Dyer CA (2003) Relationship between myelin production and dopamine synthesis in the PKU mouse brain. J Neurochem 86:615–626
Karelson E, Bogdanovic N, Garlind A, Winblad B, Zilmer K, Kullisaar T, Vihalemm T, Kairane C, Zilmer M (2001) The cerebrocortical areas in normal brain aging and Alzheimer’s disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochem Res 26:353–361
Kölker S, Ahlemeyer B, Huhne R, Mayatepek E, Krieglstein J, Hoffmann GF (2001a) Potentiation of 3-hydroxyglutarate neurotoxicity following induction of astrocytic iNOS in neonatal rat hippocampal cultures. Eur J Neurosci 13:2115–2122
Kölker S, Ahlemeyer B, Krieglstein J, Hoffmann GF (2001b) Contribution of reactive oxygen species to 3-hydroxyglutarate neurotoxicity in primary neuronal cultures from chick embryo telencephalons. Pediatr Res 50:76–82
Kuhn DM, Aretha CW, Geddes TJ (1999) Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. J Neurosci 19:10289–10294
Latini A, Scussiato K, Rosa RB, Leipnitz G, Llesuy S, Belló-Klein A, Dutra-Filho CS, Wajner M (2003a) Induction of oxidative stress by l-2-hydroxyglutaric acid in rat brain. J Neurosci Res 74:103–110
Latini A, Scussiato K, Rosa RB, Llesuy S, Belló-Klein A, Dutra-Filho CS, Wajner M (2003b) d-2-Hydroxyglutaric acid induces oxidative stress in cerebral córtex of young rats. Eur J Neurosci 17:2017–2022
Lowry OH, Rosebrough NJ, Lewis-Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mancuso C, Scapagini G, Currò D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V (2007) Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci 12:1107–1123
Martinez-Cruz F, Pozo D, Osuna C, Espinar A, Marchante C, Guerrero JM (2002) Oxidative stress induced by phenylketonuria in the rat: prevention by melatonin, vitamin E and vitamin C. J Neurosci Res 69:550–558
Matalon R, Michals-Matalon K, Bhatia G, Burlina AB, Burlina AP, Braga C, Fiori L, Giovannini M, Grechanina E, Novikov P, Grady J, Tyring SK, Guttler F (2007) Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis 30:153–158
Méndez-Alvarez E, Soto-Otero R, Herminda-Ameijeiras A, López-Martín ME, Labandeira-García JL (2001) Effect of iron and manganese on hydroxyl radical production by 6-hydroxydopamine: mediation of antioxidants. Free Radic Biol Med 31:986–998
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Möller HE, Ullrich K, Weglage J (2000) In vivo proton magnetic resonance spectroscopy in phenylketonuria. Eur J Pediatr 159:S121–S125
Möller HE, Weglage J, Bick U, Wiedermann D, Feldmann R, Ullrich K (2003) Brain imaging and proton magnetic resonance spectroscopy in patients with phenylketonuria. Pediatrics 112:1580–1583
Moyano D, Vilaseca MA, Pineda M, Campistol J, Vernet A, Póo P, Artuch R, Sierra C (1997) Tocopherol in inborn errors of intermediary metabolism. Clin Chim Acta 263:147–155
Pardridge WM (1998) Blood–brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res 23:635–644
Pérez-Severiano F, Rios C, Segovia J (2000) Striatal oxidative damage parallels the expression of a neurological phenotype in mice transgenic for the mutation of Huntington’s disease. Brain Res 862:234–237
Pietz J, Rupp A, Ebinger F, Rating D, Mayatepek E, Boesch C, Kreis R (2003) Cerebral energy metabolism in phenylketonuria: findings by quantitative in vivo 31P MR spectroscopy. Pediatr Res 53:654–662
Pratt OEA (1980) New approach to the treatment of phenylketonuria. J Ment Defic Res 24:203–217
Rech VC, Feksa LR, Dutra-Filho CS, Wyse ATS, Wajner M, Wannmacher CM (2002) Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res 27:353–357
Sarkissian CN, Scriver CR, Mamer OA (2000) Measurement of phenyllactate, phenylacetate, and phenylpyruvate by negative ion chemical ionization-gas chromatography/mass spectrometry in brain of mouse genetic models of phenylketonuria and non-phenylketonuria hyperphenylalaninemia. Anal Biochem 280:242–249
Schumacher U, Lukacs Z, Kaltschmidt C, Freudlsperger C, Schulz D, Kompisch K, Müller R, Rudolph T, Santer R, Lorke DE, Ullrich K (2008) High concentrations of phenylalanine stimulate peroxisome proliferator-activated receptor gamma: implications for the pathophysiology of phenylketonuria. Neurobiol Dis 32:385–390
Scriver CR (2001) Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 1667–1724
Shefer S, Tint GS, Jean-Guillaume D, Daikhin E, Kendler A, Nguyen LB, Yudkoff M, Dyer CA (2000) Is there a relationship between 3-hydroxy-3-methylglutaryl coenzyme a reductase activity and forebrain pathology in PKU mouse? J Neurosci Res 61:549–563
Sierra C, Vilaseca MA, Moyano D, Brandi N, Campistol J, Lambruschini N, Cambra FJ, Deulofeu R, Mira A (1998) Antioxidant status in hyperphenylalaninemia. Clin Chim Acta 276:1–9
Sierra C, Vilaseca MA, Brandi N, Artuch R, Mira A, Nieto M, Pineda M (2001) Oxidative stress in Rett syndrome. Brain Dev 23:S236–S239
Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Belló-Klein A, Giugliani R, Deon M, Vargas CR (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740:68–73
Sitta A, Barschak AG, Deon M, Terroso T, Pires R, Giugliani R, Dutra-Filho CS, Wajner M, Vargas CR (2006) Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab Brain Dis 21:287–296
Sitta A, Barschak AG, Deon M, Barden AT, Biancini GB, Vargas PR, de Souza CF, Netto C, Wajner M, Vargas CR (2009a) Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int J Dev Neurosci 27:243–247
Sitta A, Barschak AG, Deon M, de Mari JF, Barden AT, Vanzin CS, Biancini GB, Schwartz IV, Wajner M, Vargas CR (2009b) l-Carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell Mol Neurobiol 29:211–218
Smith QR, Momma S, Aoyagi M, Rapoport SI (1987) Kinetics of neutral amino acid transport across the blood–brain barrier. J Neurochem 49:1651–1658
Stoy N, Mackay GM, Forrest CM, Christofides J, Egerton M, Stone TW, Darlington LG (2005) Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem 93:611–623
Streck EL, Zugno AI, Tagliari B, Franzon R, Wannmacher CM, Wajner M, Wyse ATS (2001) Inhibition of rat brain Na+, K+-ATPase activity induced by homocysteine is probably mediated by oxidative stress. Neurochem Res 26:1195–1200
Streck EL, Vieira PS, Wannmacher CMD, Dutra-Filho CS, Wajner M, Wyse ATS (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18:147–154
Surtees R, Blau N (2000) The neurochemistry of phenylketonuria. Eur J Pediatr 159:S109–S113
van Spronsen FJ, van Rijn M, Bekhof J, Koch R, Smit PG (2001) Phenylketonuria: tyrosine supplementation in phenylalanine-restricted diets. Am J Clin Nutr 73:153–157
van Spronsen FJ, Hoeksma M, Reijngoud DJ (2009) Brain dysfunction in phenylketonuria: is phenylalanine toxicity the only possible case? J Inherit Metab Dis 32:46–51
Vargas CR, Wajner M, Sirtori LR, Goulart L, Chiochetta M, Coelho D, Latini A, Llesuy S, Belló-Klein A, Giugliani R, Deon M, Mello CF (2004) Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy. Biochim Biophys Acta 1688:26–32
Wajner M, Latini A, Wyse ATS, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27:427–448
Wilke BC, Vidailhet M, Favier A, Guillemin C, Ducros V, Arnaud J, Richard MJ (1992) Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before an after selenium supplementation. Clin Chim Acta 207:137–142
Wood B (1976) Neurological disturbance in a phenylketonuric child after discontinuation of dietary treatment. Dev Med Child Neurol 18:657–665
Acknowledgments
This work was supported by grants from CNPq, PRONEX II, FAPERGS, PROPESQ/UFRGS, FINEP research grant Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00 and INCT-EN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, C.G., Leipnitz, G., Seminotti, B. et al. Experimental Evidence that Phenylalanine Provokes Oxidative Stress in Hippocampus and Cerebral Cortex of Developing Rats. Cell Mol Neurobiol 30, 317–326 (2010). https://doi.org/10.1007/s10571-009-9455-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9455-6