Abstract
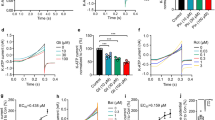
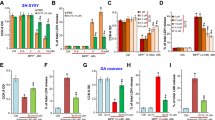
Growing evidence suggests that the astrocytic gap junctions (GJs), mainly formed by connexin 43 (Cx43), play an important role in physiological maintenance and various central nervous system (CNS) pathological conditions. However, little is known about the role of Cx43 in Parkinson’s disease (PD). In this article, we report that rotenone, a classic neurotoxin for PD, could inhibit expression of astrocytic Cx43 and gap junction permeability. ATP-sensitive potassium (KATP) channel openers, iptakalim (IPT) and diazoxide (DZ), exerted protective effect on rotenone-induced dysfunction of Cx43 and astrocyte apoptosis, which was reversed by selective mitochondrial KATP (mitoKATP) channel blocker 5-hydroxydecanoate (5-HD). Taken together, our findings reveal that rotenone-induced dysfunction of astrocytic Cx43 may be involved in the pathology of PD. Moreover, opening mitoKATP channels in astrocytes can reverse rotenone-induced dysfunction of astrocytic Cx43 and therefore protect against toxicity of rotenone on astrocytes.



Similar content being viewed by others
References
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136:317–324
Amiry-Moghaddam M, Ottersen OP (2003) The molecular basis of water transport in the brain. Nat Rev Neurosci 4:991–1001
Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P (2001) Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem 276:33369–33374
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Blandini GF, Nappi C, Tassorelli E, Martignoni E (2000) Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol 62:63–88
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9:917–924
Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA (2001) Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem 77:1601–1610
Contreras JE, Sanchez HA, Eugenin EA et al (2002) Metabolic inhibition induces opening of unapposed connexin43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99:495–500
Contreras JE, Sanchez HA, Veliz LP et al (2004) Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev 47:290–303
Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC (2000) Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev 32:45–56
Diaz-Corrales FJ, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N (2005) Rotenone induces aggregation of gamma-tubulin protein and subsequent disorganization of the centrosome: relevance to formation of inclusion bodies and neurodegeneration. Neuroscience 133:117–135
El-Fouly MH, Trosko JE, Chang CC (1987) A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res 168:422–430
Farahani R, Pina-Benabou MH, Kyrozis A, Rozental R et al (2005) Alterations in metabolism and gap junction expression may determine the role of astrocytes as ‘‘good samaritans’’ or executioners. Glia 50:351–361
Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A (2007) Glia: the fulcrum of brain diseases. Cell Death Differ 14:1324–1335
Goubaeva F, Mikami M, Giardina S, Ding B, Abe J, Yang J (2007) Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem Biophys Res Commun 352:97–103
Hamprecht B, Loffler F (1985) Primary glial cultures as a model for studying hormone action. Methods Enzymol 109:341–345
Helmuth L (2000) Pesticide causes Parkinson’s in rats. Science 290:1068
Hu LF, Wang S, Shi XR, Yao HH, Sun YH, Ding JH, Hu G (2005) ATP-sensitive potassium channel opener iptakalim protected against the cytotoxicity of MPP+ on SH-SY5Y cells by decreasing extracellular glutamate level. J Neurochem 94:1570–1579
John GR, Scemes E, Suadicani SO, Liu JS, Charles PC et al (1999) IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci USA 96:11613–11618
Kawasaki A, Hayashi T, Nakachi K, Trosko JE, Sugihara K, Kotake Y, Ohta S (2009) Modulation of connexin 43 in rotenone-induced model of Parkinson’s disease. Neuroscience 160:61–68
Kielian T (2008) Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem 106:1000–1016
Krysko DV, Leybaert L, Vandenabeele P, D’Herde K (2005) Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10:459–469
Liao CK, Wang SM, Chen YL, Wang HS, Wu JC (2010) Lipopolysaccharide-induced inhibition of connexin 43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3. Int J Biochem Cell Biol 42:762–770
Liss B, Roeper J (2001) Molecular physiology of neuronal K-ATP channels (Review). Mol Membr Biol 18:117–127
Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J (2005) K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci 8:1742–1751
Mazzanti M, Sul JY, Haydon PG (2001) Glutamate on demand: astrocytes as a ready source. Neuroscientist 7:396–405
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Mov Disord 23:474–483
Nagy JI, Rash JE (2003) Astrocyte and oligodendrocyte connexins of the glial syncytium in relation to astrocyte anatomical domains and spatial buffering. Cell Commun Adhes 10:401–406
Nagy JI, Li W, Hertzberg EL, Marotta CA (1996) Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res 717:173–178
Nakase T, Naus CC (2004) Gap junctions and neurological disorders of the central nervous system. Biochim Biophys Acta 1662:149–158
Nakase T, Fushiki S, Naus CCG (2003) Astrocytic gap junctions composed by connexin43 reduces apoptotic neuronal damage in cerebral ischemia. Stroke 34:1987–1993
Perez Velazquez JL, Frantseva MV, Naus CC (2003) Gap junctions and neuronal injury: protectants or executioners? Neuroscientist 9:5–9
Scarabelli TM, Knight R, Stephanou A et al (2006) Clinical implications of apoptosis in ischemic myocardium. Curr Probl Cardiol 31:181–264
Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7:194–206
Spray DC, Ye ZC, Ransom BR (2006) Functional connexin ‘hemichannels’: a critical appraisal. Glia 54:758–773
Tabernero A, Medina JM, Giaume C (2006) Glucose metabolism and proliferation in glia: role of astrocytic gap junctions. J Neurochem 99:1049–1061
Tai KK, Truong DD (2002) Activation of adenosine triphosphate-sensitive potassium channels confers protection against rotenone-induced cell death: therapeutic implications for Parkinson’s disease. J Neurosci Res 69:559–566
Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN et al (2001) Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol Cell Neurosci 18:671–690
Wang H (2003) Pharmacological characteristics of the novel antihypertensive drug, iptakalim hydrochloride, and its molecular mechanisms. Drug Dev Res 58:65–68
Wang S, Hu LF, Yang Y, Ding JH, Hu G (2005) Studies of ATP-sensitive potassium channels on 6-hydroxydopamine and haloperidol rat models of Parkinson’s disease: implications for treating Parkinson’s disease? Neuropharmacology 48:984–992
Wang S, Hu LF, Zhang Y, Sun T, Sun YH, Liu SY et al (2006) Effects of systemic administration of iptakalim on extracellular neurotransmitter levels in the striatum of unilateral 6-hydroxydopamine-lesioned rats. Neuropsychopharmacology 31:933–940
Xie W, Wang H, Ding J, Wang H, Hu G (2005) Anti-proliferating effect of iptakalim, a novel KATP channel opener, in cultured rabbit pulmonary arterial smooth muscle cells. Eur J Pharmacol 511:81–87
Yang Y, Liu X, Ding JH, Sun J, Long Y, Wang F et al (2004) Effects of iptakalim on rotenone-induced cytotoxicity and dopamine release from PC12 cells. Neurosci Lett 366:53–57
Yang Y, Liu X, Long Y, Wang F, Ding JH, Liu SY et al (2005) Systematic administration of iptakalim, an ATP-sensitive potassium channel opener, prevents rotenone-induced motor and neurochemical alterations in rats. J Neurosci Res 80:442–449
Yang J, Hu LF, Liu X, Zhou F, Ding JH, Hu G (2006a) Effects of iptakalim on extracellular glutamate and dopamine levels in the striatum of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Life Sci 78:1940–1944
Yang Y, Liu X, Long Y, Wang F, Ding JH, Liu SY et al (2006b) Activation of mitochondrial ATP-sensitive potassium channels improves rotenone-related motor and neurochemical alterations in rats. Int J Neuropsychopharmacol 9:51–61
Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B (1999) Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol 514:327–341
Zhang S, Zhou F, Ding JH, Zhou XQ, Sun XL, Hu G (2007) ATP-sensitive potassium channel opener iptakalim protects against MPP+-induced astrocytic apoptosis via mitochondria and MAPK signal pathways. J Neurochem 103:569–579
Zhou F, Wu JY, Sun XL, Yao HH, Ding JH, Hu G (2007) Iptakalim alleviates rotenone-induced degeneration of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology 32:2570–2580
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (No. 30625038), and the National Key Basic Research Program of China (No. 2011CB504103 and No. 2009CB521906).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Liang, R., Zhou, F. et al. Reversal of Rotenone-Induced Dysfunction of Astrocytic Connexin43 by Opening Mitochondrial ATP-Sensitive Potassium Channels. Cell Mol Neurobiol 31, 111–117 (2011). https://doi.org/10.1007/s10571-010-9560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9560-6