Abstract
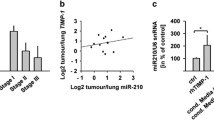
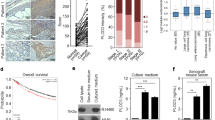
The “protease web”, representing the network of proteases, their inhibitors, and effector molecules, arises as a pivotal determinant of tissue homeostasis. Imbalances of this network, for instance caused by elevated host levels of tissue inhibitor of metalloproteinases-1 (TIMP-1), have been shown to increase the susceptibility of target organs to scattered metastasis by inducing the hepatocyte growth factor (HGF) pathway. Increased expression of the hypoxia-inducible factor-1α-subunit (HIF-1α) is also associated with tumour progression and is also known to induce HGF-signaling via up-regulation of the HGF-receptor Met, namely under canonical stress conditions like lack of oxygen. Here, we aimed to identify a possible metastasis-promoting connection between TIMP-1, HIF-1α, and HGF-signaling. We found that HIF-1α and HIF-1-signaling were increased during liver metastasis of L-CI.5s T-lymphoma cells in TIMP-1 overexpressing syngeneic DBA/2 mice. In vitro, exposure of L-CI.5s cells to recombinant TIMP-1 revealed that TIMP-1 itself was able to induce HIF-1α and HIF-1-signaling. Knock-down of HIF-1α identified tumour cell-derived HIF-1α as mediator of this TIMP-1-induced invasiveness in vitro. In vivo, HIF-1α knock-down significantly impaired Met expression as well as Met phosphorylation and inhibited scattered liver metastasis. Furthermore, HGF-dependent TIMP-1-promoted Met phosphorylation and HGF-dependent TIMP-1-induced invasiveness in vitro was mediated by HIF-1α. We conclude that elevated levels of TIMP-1 in the microenvironment of tumour cells can promote metastasis by inducing HIF-1α-dependent HGF-signaling. This connection between a protease inhibitor (TIMP-1) and a classically stress-related factor (HIF-1α) is a so far undiscovered impact of the “protease web” on tissue homeostasis with important implications for metastasis.






Similar content being viewed by others
Abbreviations
- Ad:
-
Adenovirus
- BSA:
-
Bovine serum albumine
- CAIX:
-
Carboanhydrase IX
- DAB:
-
3,3′-Diaminobenzidine
- DAPI:
-
4′,6-Diamidine-2-phenylindole
- HEK:
-
Human embryonic kidney cell
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia-inducible factor
- HRE:
-
Hypoxia-responsive element
- HRP:
-
Horseradish peroxidase
- i.v.:
-
Intraveneous
- Met:
-
Hepatocyte growth factor receptor
- MMP:
-
Matrix metalloproteinase
- NT:
-
Non-template
- PBS:
-
Phosphate-buffered saline
- Pfu:
-
Plaque-forming unit
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- rec:
-
Recombinant
- SDS-PAGE:
-
Sodiumdodecylsulfate polyacrylamide gel electrophoresis
- SEM:
-
Standard error mean
- sh:
-
Small hairpin
- TBS:
-
2-Amino-2-(hydroxymethyl)-propane-1,3-diole-buffered saline
- TIMP:
-
Tissue inhibitor of metalloproteinases
- X-Gal:
-
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside
References
Kaplan RN, Rafii S, Lyden D (2006) Preparing the “soil”: the premetastatic niche. Cancer Res 66(23):11089–11093
Overall CM, Dean RA (2006) Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev 25(1):69–75
Kopitz C et al (2007) Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res 67(18):8615–8623
Schrotzlmair F et al. (2009) Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by host-derived urokinase-type plasminogen activator. J Cell Mol Med. doi:10.1111/j.1582-4934.2009.00951.x. Accessed 23 Oct 2009
Benvenuti S, Comoglio PM (2007) The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol 213(2):316–325
Pennacchietti S et al (2003) Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3(4):347–361
Mendoza M, Khanna C (2009) Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol 41(7):1452–1462
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4(11):891–899
Pouyssegur J, Dayan F, Mazure NM (2006) Hypoxia signaling in cancer and approaches to enforce tumour regression. Nature 441(7092):437–443
Chan DA, Giaccia AJ (2007) Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev 26(2):333–339
Brahimi-Horn MC, Pouyssegur J (2009) HIF at a glance. J Cell Sci 122(Pt 8):1055–1057
Wykoff CC et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60(24):7075–7083
Aiello L et al (1979) Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293). Virology 94(2):460–469
Krüger A, Schirrmacher V, von Hoegen P (1994) Scattered micrometastases visualized at the single-cell level: detection and re-isolation of lacZ-labeled metastasized lymphoma cells. Int J Cancer 58(2):275–284
Gerg M et al (2008) Distinct functionality of tumor cell-derived gelatinases during formation of liver metastases. Mol Cancer Res 6(3):341–351
Jones N, Shenk T (1978) Isolation of deletion and substitution mutants of adenovirus type 5. Cell 13(1):181–188
Hitt M et al (1995) Techniques for human adenovirus vector construction and characterization. In: Adolph KW (ed) Viral gene techniques. Academic Press, San Diego, pp 13–30
Arlt M et al (2002) Increase in gelatinase-specificity of matrix metalloproteinase inhibitors correlates with antimetastatic efficacy in a T-Cell lymphoma model. Cancer Res 62(19):5543–5550
Kopitz C et al (2005) Reduction of experimental human fibrosarcoma lung metastasis in mice by adenovirus-mediated cystatin C overexpression in the host. Cancer Res 65(19):8608–8612
Kopitz C et al (2008) Plasminogen activator inhibitor-2, but not cystatin C, inhibits the prometastatic activity of tissue inhibitor of metalloproteinases-1 in the liver. Hum Gene Ther 19(10):1039–1049
Flannelly J et al (2002) Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthr Cartil 10(9):722–733
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Schelter F et al. (2010) Identification of a survival-independent metastasis-enhancing role of hypoxia-inducible factor-1{alpha} with a hypoxia-tolerant tumor cell line. J Biol Chem. doi: 10.1074/jbc.M110.140608
Acknowledgments
The author thank Katja Honert, Mareike Lehnhoff, Tina Krause, and Dr Susanne Schaten (all from Institut für Experimentelle Onkologie und Therapieforschung des Klinikums rechts der Isar, Technische Universität München, Munich, Germany) for their expert technical assistance. For financial support, the authors thank the European Union Research Framework Programme 7, project HEALTH-2007-201279/Microenvimet (to Achim Krüger) and project HEALTH-F2-2009-222741/Metoxia (to Agnes Görlach), and the Deutsche Forschungsgemeinschaft, grant KR2047/1-1 (to Achim Krüger).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schelter, F., Halbgewachs, B., Bäumler, P. et al. Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by hypoxia-inducible factor-1α. Clin Exp Metastasis 28, 91–99 (2011). https://doi.org/10.1007/s10585-010-9360-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-010-9360-x