Abstract
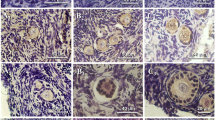
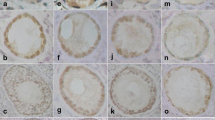
The synergetic process of folliculogenesis is mainly regulated by GDF-9 and BMP-15 as well as their receptors, such as BMPR2, TβR1 and BMPR1B. Expressions of these factors and the receptors are significant different among species. This study was designed to detect expression of GDF-9, BMP-15 and their receptors in mouse, porcine and human healthy follicles by immunohistochemistry. Three ages of human ovary were studied according to ovarian developmental schedule, i.e. gestational week (GW) 16, puberty (14 year-old) and adult (40 year-old). The results showed that both GDF-9 and BMP-15 were detectable in oocytes from primary follicles onward, besides, BMP-15 also presented in granulosa cells (GCs) and follicular follicle of mature follicles in mouse. However, they were maintained in oocytes and GCs from primordial to mature follicles in porcine except that GDF-9 was undetectable in GCs of mature follicles. For human ovary, GDF-9 presented in oocytes of primordial follicles in all samples, whereas BMP-15 was only observed in primordial follicle of adult ovary. Receptors, BMPR2, TβR1 and BMPR1B were found in oocytes and GCs of all follicles in mouse and porcine. In human, they were stained in oocytes from primordial follices but BMPR1B was not expressed in pubertal primordial follicles. Furthermore, we found that GDF-9, BMP-15 and three receptors distributed in adult corpus lutea. Collectively, our studies suggested that GDF-9, BMP-15 and their receptors might correlate with primordial follicular recruitment in pig and human. Positive expression of the receptors (BMPR2, TβR1 and BMPR1B)in primordial follicles of mouse ovaries indicated that these receptors might interact with others ligands besides GDF-9 and BMP-15 to regulate primordial follicular activity in mouse. Moreover, presence of GDF-9 in oocytes and BMP-15 in oocytes and GCs of mature follicles from mice and porcine elucidated coordinated roles of GDF-9 and BMP-15 in cumulus oophorus expansion. Additionally, expression of these factors in adult human corpus lutea suggested they play roles in corpus luteum activity.





Similar content being viewed by others
References
Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppä L, Louhio H, Tuuri T, Sjöberg J, Bützow R, Hovata O, Dale L, Ritvos O (1999) Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during Early Folliculogenesis. J Clin Endocrinol Metab 84(8):2744–2750
Di Pasquale E, Beck-Peccoz P, Persani L (2004) Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP-15) gene. Am J Hum Genet 75(1):106–111
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383(6600):531–535
Eckery DC, Whale LJ, Lawrence SB, Wylde KA, McNatty KP, Juengel JL (2002) Expression of mRNA encoding growth differentiation factor 9 and bone morphogenetic protein 15 during follicular formation and growth in a marsupial, the brushtail possum (Trichosurus vulpecula). Mol Cell Endocrinol 192(1–2):115–126
Edwards SJ, Reader KL, Lun S, Western A, Lawrence S, McNatty KP, Juengel JL (2008) The cooperative effect of growth and differentiation factor-9 and bone morphogenetic protein (BMP)-15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology 149(3):1026–1030
Erickson GF, Shimasaki S (2003) The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol 1(9):1–20
Feary ES, Juengel JL, Smith P, French MC, O’Connell AR, Lawrence SB, Galloway SM, Davis GH, McNatty KP (2007) Patterns of expression of messenger RNAs encoding GDF-9, BMP-15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in ewes carrying the woodlands FecX2 W mutation. Biol Reprod 77(6):990–998
Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG (2006) Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci 119(Pt 18):3811–3821
Guéripel X, Brun V, Gougeon A (2006) Oocyte bone morphogenetic protein 15, but not growth differentiation factor 9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion. Biol Reprod 75(6):836–843
Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM (2004) Mutations in the genes for oocyte-derived growth factors GDF-9 and BMP-15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 70(4):900–909
Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O (2002) Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab 87(1):316–321
Jayawardana BC, Shimizu T, Nishimoto H, Kaneko E, Tetsuka M, Miyamoto A (2006) Hormonal regulation of expression of growth differentiati factor-9 receptor type I and II genes in the bovine ovarian follicle. Reproduction 131(3):545–553
Juengel JL, McNatty KP (2005) The role of proteins of the transforming growth factor-β superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 11(2):143–160
Juengel JL, Hudson NL, Berg M, Hamel K, Smith P, Lawrence SB, Whiting L, McNatty KP (2009) Effects of active immunization against growth differentiation factor 9 and/or bone morphogenetic protein 15 on ovarian function in cattle. Reproduction 138(1):107–114
Knight PG, Glister C (2006) TGF-β superfamily members and ovarian follicle development. Reproduction 132(2):191–206
Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss AC, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA (2006) Mutations and sequence variants in GDF-9 and BMP-15 in patients with premature ovarian failure. Eur J Endocrinol 154(5):739–744
Li HK, Kuo TY, Yang HS, Chen LR, Li SS, Huang HW (2008a) Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim Reprod Sci 103(3–4):312–322
Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM (2008b) Redundant Roles of SMAD2 and SMAD3 in Ovarian GCsIn Vivo. Mol Cell Biol 28(23):7001–7011
Margulis S, Abir R, Felz C, Nitke S, Krissi H, Fisch B (2009) Bone morphogenetic protein 15 expression in human ovaries from fetuses, girls, and women. Fertil Steril 92(5):1666–1673
Martins FS, Celestino JJ, Saraiva MV, Matos MH, Bruno JB, Rocha-Junior CM, Lima-Verde IB, Lucci CM, Báo SN, Figueiredo JR (2008) Growth and differentiation factor-9 stimulates activation of goat primordial follicles in vitro and their progression to secondary follicles. Reprod Fertil Dev 20(8):916–924
Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJ (2004) Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol 18(3):653–665
McGrath SA, Esquela AF, Lee SJ (1995) Oocyte-specific expression of growth differentiation factor-9. Mol Endocrinol 9(1):131–136
McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL (2008) The proregion of mouse BMP-15 regulates the cooperative interactions of BMP-15 and GDF-9. Biol Reprod 79(5):889–896
McNatty KP, Hudson NL, Whiting L, Reader KL, Lun S, Western A, Heath DA, Smith P, Moore LG, Juengel JL (2007) The effects of immunizing sheep with different BMP-15 or GDF-9 peptide sequences on ovarian follicular activity and ovulation rate. Biol Reprod 76(4):552–560
Montgomery GW, Zhao ZZ, Marsh AJ, Mayne R, Treloar SA, James M, Martin NG, Boomsma DI, Duffy DL (2004) A deletion mutation in GDF-9 in sisters with spontaneous DZ twins. Twin Res 7(6):548–555
Moore KL, Persaud TVN, Torchia MG (2008) The developing human: clinically oriented embryology, 8th edn. Elsevier, Amsterdam
Nilsson EE, Skinner MK (2003) Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 69(4):1265–1272
Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK (2009) Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology 150(6):2740–2748
Paradis F, Novak S, Murdoch G, Dyck MK, Dixon WT, Foxcroft GR (2009) Temporal regulation of BMP2, BMP6, BMP-15, GDF-9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 138(1):115–129
Peng X, Yang M, Wang L, Tong C, Guo Z (2010) In vitro culture of sheep lamb ovarian cortical tissue in a sequential culture medium. J Assist Reprod Genet 27(5):247–257
Quinn RL, Shuttleworth G, Hunter MG (2004) Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary. J Anat 205(1):15–23
Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, Nelson LM, Beck-Peccoz P, Persani L (2009) BMP-15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat 30(5):804–810
Sadeu JC, Adriaenssens T, Smitz J (2008) Expression of growth differentiation factor 9, bone morphogenetic protein 15, and anti-Mullerian hormone in cultured mouse primary follicles. Reproduction 136(2):195–203
Shimasaki S, Moore RK, Otsuka F, Erickson GF (2004) The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25(1):72–101
Shimizu T, Miyahayashi Y, Yokoo M, Hoshino Y, Sasada H, Sato E (2004) Molecular cloning of porcine growth differentiation factor 9 (GDF-9) cDNA and its role in early folliculogenesis: direct ovarian injection of GDF-9 gene fragments promotes early folliculogenesis. Reproduction 128(5):537–543
Silva JR, van den Hurk R, van Tol HT, Roelen BA, Figueiredo JR (2005) Expression of growth differentiation factor 9 (GDF-9), bone morphogenetic protein 15 (BMP-15), and BMP receptors in the ovaries of goats. Mol Reprod Dev 70(1):11–19
Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ (2008) Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP-15 and GDF-9 control cholesterol biosynthesis in cumulus cells. Development 135(1):111–121
Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF (2002) Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab 87(3):1337–1344
Vitt UA, Mazerbourg S, Klein C, Hsueh AJ (2002) Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod 67(2):473–480
Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM (2001) The type I BMP receptor BmprIB is essential for female reproductive function. PNAS 98(14):7994–7999
Yoshino O, McMahon HE, Sharma S, Shimasaki S (2006) A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. PNAS 103(28):10678–10683
Zhu G, Guo B, Pan D, Mu Y, Feng S (2008) Expression of bone morphogenetic proteins and receptors in porcine cumulus–oocyte complexes during in vitro maturation. Anim Reprod Sci 104(2–4):275–283
Acknowledgments
This study was supported by the High-Tech Research and Development (863) Programme “Studies of efficiently bisection, cryopreservation and molecular label of domestic animal embryos” (2008AA101007) and the Specialist Funding from the Education Department of Heilongjiang Province (1153-NCET-007). The authors thank the Department of Forensic Medicine of Harbin Medical University for providing materials. The experiments performed in this study complied with the current laws and regulations in the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Z. Sun and L. Lei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, R.Z., Lei, L., Cheng, L. et al. Expression of GDF-9, BMP-15 and their receptors in mammalian ovary follicles. J Mol Hist 41, 325–332 (2010). https://doi.org/10.1007/s10735-010-9294-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-010-9294-2