Abstract
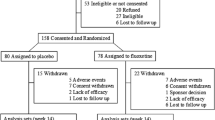
Efficacy and safety of 2 risperidone doses were evaluated in children and adolescents with autism. Patients (N = 96; 5–17 years), received risperidone (low-dose: 0.125 mg/day [20 to <45 kg], 0.175 mg/day [>45 kg] or high-dose: 1.25 mg/day [20 to <45 kg], 1.75 mg/day [>45 kg]) or placebo. Mean baseline (range 27–29) to endpoint change in Aberrant Behavior Checklist-Irritability (primary endpoint) was significantly greater in the high-dose—(−12.4 [6.5]; p < 0.001), but not low-dose (−7.4 [8.1]; p = 0.164) group, versus placebo (−3.5 [10.7]). Clinical Global Impressions-Severity and Children’s Yale-Brown Obsessive Compulsive Scale scores improved significantly only in the high-dose group, consistent with ABC-I results. Somnolence, sedation and increased appetite occurred more frequently in high-versus low-dose groups. Overall, increased appetite occurred most frequently.



Similar content being viewed by others
References
Aman, M. G., Arnold, L. E., McDougle, C. J., Vitiello, B., Scahill, L., Davies, M., et al. (2005). Acute and long-term safety and tolerability of risperidone in children with autism. Journal of Child and Adolescent Psychopharmacology, 15(6), 869–884.
Aman, M. G., & Singh, N. N. (1994). Aberrant behavior checklist—community. (supplementary manual). East Aurora, NY: Slosson Educational Publication.
Aman, M. G., Singh, N. N., Stewart, A. W., & Field, C. J. (1985a). The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89(5), 485–491.
Aman, M. G., Singh, N. N., Stewart, A. W., & Field, C. J. (1985b). Psychometric characteristics of the Aberrant Behavior Checklist. American Journal of Mental Deficiency, 89(5), 492–502.
Aman, M. G., Vinks, A. A., Remmerie, B., Mannaert, E., Ramadan, Y., Masty, J., et al. (2007). Plasma pharmacokinetics characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorder. Clinical Therapeutics, 29, 1476–1486.
Anderson, G. M., Scahill, L., McCracken, J. T., McDougle, C. J., Aman, M. G., Tierney, E., et al. (2007). Effect of short- and long-term risperidone treatment on prolactin levels in children with autism. Biological Psychiatry, 61(4), 545–550.
Arnold, L. E., Vitiello, B., Mcdougle, C., Scahill, L., Shah, B., Gonzalez, N. M., et al. (2003). Parent-defined target symptoms respond to risperidone in RUPP autism study: Customer approach to clinical trials. Journal of the American Academy of Child and Adolescent Psychiatry, 42(12), 1443–1450.
Barnes, T. R. (1989). A rating scale for drug-induced akathisia. British Journal of Psychiatry, 154, 672–676.
Findling, R. L., Kusumakar, V., Daneman, D., Moshang, T., De Smedt, G., & Binder, C. (2003). Prolactin levels during long-term risperidone treatment in children and adolescents. Journal of Clinical Psychiatry, 64(11), 1362–1369.
Freitag, C. M. (2007). The genetics of autistic disorders and its clinical relevance: A review of the literature. Molecular Psychiatry, 12(1), 2–22.
Gagliano, A., Germano, E., Pustorino, G., Impallomeni, C., D’arrigo, C., Calamoneri, F., et al. (2004). Risperidone treatment of children with autistic disorder: Effectiveness, tolerability, and pharmacokinetic implications. Journal of Child and Adolescent Psychopharmacology, 14(1), 39–47.
Guy, W. (Ed.). (1976a). Clinical Global Impression (CGI). ECDEU assessment manual for psychopharmacology. National Institute of Mental Health (pp. 218–222). Rockville, Maryland: Welfare publication.
Guy, W. (Ed.). (1976b). AIMS. Abnormal Involuntary Movement Scale. ECDEU Assessment for Psychopharmacology. Rev. Ed, National Institute of Mental Health (pp. 537–543). Rockville, Maryland: Welfare publication.
Libbey, J. E., Sweeten, T. L., McMahon, W. M., & Fujinami, R. S. (2005). Review: Autistic disorder and viral infections. Journal of Neurovirology, 11, 1–10.
Lieberman, J. A. (2004). Metabolic changes associated with antipsychotic use. Prim Care Campanion. Journal of Clinical Psychiatry, 6(2), 8–13.
Limperopoulos, C., Bassan, H., Gauvreau, K., Robertson, R. L., Jr, Sullivan, N. R., Benson, C. B., et al. (2007). Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics, 120(3), 584–593.
Malone, R. P., Maislin, G., Choudhury, M. S., Gifford, C., & Delaney, M. A. (2002). Risperidone treatment in children and adolescents with autism: Short- and long-term safety and effectiveness. Journal of the American Academy of Child and Adolescent Psychiatry, 41(2), 140–147.
Mc Dougle, C. J., Stigler, K. A., & Posey, D. J. (2003). Treatment of aggression in children and adolescent with autism and conduct disorder. Journal of Clinical Psychiatry, 64(4), 16–25.
McCracken, J. T., Mcgough, J., Shah, B., Cronin, P., Hong, D., Aman, M. G., et al. (2002). Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine, 347(5), 314–321.
Nicolson, R., Awad, G., & Sloman, L. (1998). An open trial of risperidone in young autistic children. Journal of the American Academy of Child and Adolescent Psychiatry, 37(4), 372–376.
Pandina, G. J., Bossie, C. A., Youssef, E., Zhu, Y., & Dunbar, F. (2007). Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. Journal of Autism and Developmental Disorders, 37(2), 367–373.
Persico, A. M., & Bourgeron, T. (2006). Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends in Neurosciences, 29(7), 349–358.
Reyes, M., Buitelaar, J., Toren, P., Augustyns, I., & Eerdekens, M. (2006). A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. American Journal of Psychiatry, 163, 402–410.
Risperdal® Prescribing Information (revised 2012). http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020272s065,020588s053,021444s041lbl.pdf.
RUPP. (2005). Research units on pediatric psychopharmacology autism network. Risperidone treatment of autistic disorder: Longer-term benefits and blinded discontinuation after 6 months. American Journal of Psychiatry, 162, 1361–1369.
Scahill, L., Riddle, M. A., McSwiggin-Hardin, M., Ort, S. I., King, R. A., Goodman, W. K., et al. (1997). Children’s yale-brown obsessive compulsive scale: Reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry, 36(6), 844–852.
Schultz, R. T. (2005). Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Society for Developmental Neuroscience, 23, 125–141.
Shea, S., Turgay, A., Carroll, A., Schulz, M., Orlik, H., Smith, I., et al. (2004). Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics, 114(5), e634–e641.
Simpson, G. M., & Angus, J. W. (1970). A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. Supplementum, 212, 11–19.
Szpir, M. (2006). Tracing the origins of autism: A spectrum of new studies. Environmental Health Perspectives, 114(7), A412–A418.
Thyssen, A., Vermeulen, A., Fuseau, E., Fabre, M., & Mannaert, E. (2010). Population pharmacokinetics of oral risperidone in children, adolescents and adults with psychiatric disorders. Clinical Pharmacokinetics, 49(7), 465–478.
Troost, P. W., Lahuis, B. E., Steenhuis, M. P., Ketelaars, C. E. J., Buitelaar, J. K., & Van England, H. (2005). Long-trem effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. Journal of the American Academy of Child and Adolescent Psychiatry, 44(11), 1137–1144.
Tschoner, A., Engl, J., Lamier, M., Kaser, S., Rettenbacher, M., Fleischhacker, J. R., et al. (2007). Review: Metabolic side effect of antipsychotic medication. International Journal of Clinical Practice, 61(8), 1356–1370.
Turgay, A., Binder, C., Snyder, R., & Fisman, S. (2002). Long-term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics, 110(3), 1–12.
Acknowledgments
This study was funded by Johnson & Johnson Pharmaceutical Research & Development, LLC. Dr. Ananya Chikramane (SIRO Clinpharm Pvt. Ltd.) provided writing assistance and Dr. Wendy P. Battisti (Janssen Research & Development, LLC) provided additional editorial support for this manuscript. We thank Dr. Joseph Palumbo (Janssen Research & Development, LLC) for his contributions to study design and review of the manuscript, Dr. Vivek Kusamakar, in memoriam, for his significant contributions to the study, and Ms. Andrea Davis (Janssen Research & Development, LLC), the clinical trial manager for the study. The authors also thank the study participants, without whom this study would never have been accomplished and the following investigators for their participation in this study: United States: Attalla, Ashraf, M.D.; Baber, Riaz, M.D.; Bregman, Joel, M.D.; Chueh, Daniel, M.D.; Djukic, Aleksandra, M.D.; Ginsberg, Lawrence, M.D.; Greenhill, Laurence, M.D.; Hagerman, Randi, M.D.; Handal, Nelson, M.D.; Holloway, Willis, M.D.; Kolevzon, Alexander, M.D.; Lerman, Mark, M.D.; Malone, Richard P., M.D.; Marsella, Gregory, M.D.; Melmed, Raun, M.D.; Padilla, Americo, M.D.; Quintana, Humberto, M.D.; Robb, Adelaide S., M.D; Schexnayder, Donald, M.D.; Yadalam, Kashinath, M.D.
Conflict of Interest
Dr. Aman was an investigator for this study and has received research support or been a consultant for Janssen Research & Development, LLC, Bristol-Myers Squibb, Pfizer, Forest Research, and Hoffman La Roche. Drs. Ness, Singh, Hough and Kent and Mr. Karcher are employees of Janssen Research & Development, LLC. Drs. Ning and Kushner were employed by Janssen Research & Development, LLC during the design and conduct of this study. Dr. Ning is currently employed by Purdue Pharma and Dr. Kushner is at CFG Health Systems, L.L.C. All authors met ICMJE criteria and all those who fulfilled those criteria are listed as authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Registration: This phase-4 study is registered at ClinicalTrials.gov (NCT00576732).
Rights and permissions
About this article
Cite this article
Kent, J.M., Kushner, S., Ning, X. et al. Risperidone Dosing in Children and Adolescents with Autistic Disorder: A Double-Blind, Placebo-Controlled Study. J Autism Dev Disord 43, 1773–1783 (2013). https://doi.org/10.1007/s10803-012-1723-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1723-5