Abstract
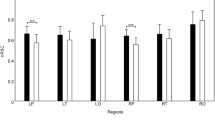
Examination of resting state brain activity using electrophysiological measures like complexity as well as functional connectivity is of growing interest in the study of autism spectrum disorders (ASD). The present paper jointly examined complexity and connectivity to obtain a more detailed characterization of resting state brain activity in ASD. Multi-scale entropy was computed to quantify the signal complexity, and synchronization likelihood was used to evaluate functional connectivity (FC), with node strength values providing a sensor-level measure of connectivity to facilitate comparisons with complexity. Sensor level analysis of complexity and connectivity was performed at different frequency bands computed from resting state MEG from 26 children with ASD and 22 typically developing controls (TD). Analyses revealed band-specific group differences in each measure that agreed with other functional studies in fMRI and EEG: higher complexity in TD than ASD, in frontal regions in the delta band and occipital-parietal regions in the alpha band, and lower complexity in TD than in ASD in delta (parietal regions), theta (central and temporal regions) and gamma (frontal-central boundary regions); increased short-range connectivity in ASD in the frontal lobe in the delta band and long-range connectivity in the temporal, parietal and occipital lobes in the alpha band. Finally, and perhaps most strikingly, group differences between ASD and TD in complexity and FC appear spatially complementary, such that where FC was elevated in ASD, complexity was reduced (and vice versa). The correlation of regional average complexity and connectivity node strength with symptom severity scores of ASD subjects supported the overall complementarity (with opposing sign) of connectivity and complexity measures, pointing to either diminished connectivity leading to elevated entropy due to poor inhibitory regulation or chaotic signals prohibiting effective measure of connectivity.









Similar content being viewed by others
References
Abasolo, D., Hornero, R., et al. (2005). Analysis of regularity in the EEG background activity of alzheimer’s disease patients with approximate entropy. Clinical Neurophysiology, 116(8), 1826–1834.
APA. (1994). Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Press.
APA. (2000). DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders. DC: Washington. American Psychiatric Association.
Assaf, M., Jagannathan, K., et al. (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage, 53(1), 247–256.
Barttfeld, P., Wicker, B., et al. (2011). A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia, 49(2), 254–263.
Basar, E., Basar-Eroglu, C., et al. (2001). Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology, 39(2–3), 241–248.
Blume, W. T. (2006). Drug effects on EEG. Journal of Clinical Neurophysiology, 23(4), 306–311.
Bosl, W., Tierney, A., et al. (2011). EEG complexity as a biomarker for autism spectrum disorder risk. BMC Medicine, 9, 18.
Breakspear, M. (2006). The nonlinear theory of schizophrenia. Australian and New Zealand Journal of Psychiatry, 40(1), 20–35.
Bressler, S. L. (1995). Large-scale cortical networks and cognition Brain Research. Brain Research Reviews, 20(3), 288–304.
Calhoun, V., Kiehl, K., et al. (2008). Modulation of temporally coherent brain networks estimated using ica at rest and during cognitive tasks. Human Brain Mapping, 29(7), 828–838.
Cantor, D. S., Thatcher, R. W., et al. (1986). Computerized EEG analyses of autistic children. Journal of Autism and Developmental Disorders, 16, 169–187.
Castelli, F., Frith, C., et al. (2002). Autism, asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain, 125, 1839–1849.
Catarino, A., Churches, O., et al. (2011). Atypical EEG complexity in autism spectrum conditions: A multiscale entropy analysis. Clinical Neurophysiology, 122(12), 2375–2383.
Coben, R., Clarke, A. R., et al. (2008). EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology, 119(5), 1002–1009.
Collins, A. L., Ma, D., et al. (2006). Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics, 7(3), 167–174.
Constantino, J., & Gruber, C. P. (2005). Social Responsiveness Scale. CA, Western Psychological Services: Los Angeles.
Cornew, L., Roberts, T. P., et al. (2012). Resting-state oscillatory activity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(9), 1884–1894.
Costa, M., Ary, L., et al. (2005). Multiscale entropy analysis of biological signals. Physical Review E, 71, 021906.
Costa, M., Goldberger, A. L., et al. (2002). Multiscale entropy analysis of complex physiologic time series. Physical Review Letters, 89(6), 068102.
Costa, M. D., Peng, C. K., et al. (2008). Multiscale analysis of heart rate dynamics: Entropy and time irreversibility measures. Cardiovascular Engineering, 8(2), 88–93.
Courchesne, E., & Pierce, K. (2005). Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology, 15(2), 225–230.
Critchley, H. D., Daly, E. M., et al. (2000). The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain, 123(Pt 11), 2203–2212.
DeLorey, T. M., Sahbaie, P., et al. (2008). Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behavioural Brain Research, 187(2), 207–220.
Dominguez, L. G., Velazquez, J. L., et al. (2013). A model of functional brain connectivity and background noise as a biomarker for cognitive phenotypes: Application to autism. PLoS ONE, 8(4), e61493.
Foley Nicpon, M., Doobay, A. F., et al. (2010). Parent, teacher, and self perceptions of psychosocial functioning in intellectually gifted children and adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40, 1028–1038.
Friston, K. J. (1996). Theoretical neurobiology and schizophrenia. British Medical Bulletin, 52(3), 644–655.
Friston, K. (2002). Beyond phrenology: What can neuroimaging tell us about distributed circuitry? Annual Review of Neuroscience, 25, 221–250.
Friston, K. J., Tononi, G., et al. (1995). Characterising the complexity of neuronal interactions. Human Brain Mapping, 3(4), 302–314.
Ghanbari, Y., Bloy, L., et al. (2012). Dominant component analysis of electrophysiological connectivity networks. In N. Ayache, H. Delingette, P. Golland, & K. Mori Nice (Eds.), International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2012), Part III. LNCS (pp. 231–238). Heidelberg: Springer.
Ghanbari, Y., Smith, A. R., et al. (2013). Connectivity subspace learning for pathology and developmental variations. International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2013), Nagoya, Japan. LNCS (pp. 90–97). Heidelberg: Springer.
Gidley Larson, J. C., & Mostofsky, S. H. (2006). Motor deficits in autism. In R. Tuchman & I. Rapin (Eds.), Autism: a neurological disorder of early brain development (pp. 231–247). London: MacKeith Press.
Harada, M., Taki, M. M., et al. (2010). Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. Journal of Autism and Developmental Disorders, 41, 447–454.
Hornero, R., Abasolo, D., et al. (2009). Nonlinear analysis of electroencephalogram and magnetoencephalogram recordings in patients with Alzheimer’s disease. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 367, 317–336.
Hughes, J. R. (2008). Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy and Behavior, 13(1), 25–31.
Just, M. A., Cherkassky, V. L., et al. (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain, 127(Pt 8), 1811–1821.
Just, M. A., Cherkassky, V. L., et al. (2007). Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex, 17(4), 951–961.
Kaiser, M. D., Delmolino, L., et al. (2010). Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research, 3, 191–195.
Khan, S., Gramfort, A., et al. (2013). Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Sciences of USA, 110, 3107–3112.
Klimesch, W., Schack, B., et al. (2005). The functional significance of theta and upper alpha oscillations. Experimental Psychology, 52(2), 99–108.
Kopell, N., Ermentrout, G. B., et al. (2000). Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences of USA, 97(4), 1867–1872.
Krug, D., & Arick, J. R. (2003). Krug Asperger’s Disorder Index. Los Angeles: Western Psychological Services.
Lord, C., Risi, S., et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223.
Monk, C. S., Peltier, S. J., et al. (2009). Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage, 47(2), 764–772.
Montez, T., Linkenkaer-Hansen, K., et al. (2006). Synchronization likelihood with explicit time-frequency priors. Neuroimage, 33(4), 1117–1125.
Murias, M., Webb, S. J., et al. (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62(3), 270–273.
Nicoll, A., Larkman, A., et al. (1993). Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. Journal of Physiology, 468, 693–710.
Orekhova, E. V., Stroganova, T. A., et al. (2007). Excess of high frequency electroencephalogram oscillations in boys with autism. Biological Psychiatry, 62, 1022–1029.
Paakki, J. J., Rahko, J., et al. (2010). Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Research, 1321, 169–179.
Pantev, C., Makeig, S., et al. (1991). Human auditory evoked gamma-band magnetic fields. Proceedings of the National Academy of Sciences of USA, 88(20), 8996–9000.
Park, J. H., Kim, S., et al. (2007). Multiscale entropy analysis of EEG from patients under different pathological conditions. Fractals, 15(04), 399–404.
Perkins, T., Stokes, M., et al. (2010). Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience, 17(10), 1239–1243.
Pincus, S. M. (1991). Approximate entropy as a measure of system-complexity. Proceedings of the National Academy of Sciences of the United States of America, 88(6), 2297–2301.
Pollonini, L., Patidar, U., et al. (2010). Functional connectivity networks in the autistic and healthy brain assessed using Granger causality. Conference Proceeding IEEE Engineering in Medicine and Biology Society (EMBS). pp 1730–1733.
Ramoz, N., Reichert, J. G., et al. (2004). Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. American Journal of Psychiatry, 161(4), 662–669.
Richman, Joshua S., Lake, Douglas E., et al. (2004). Sample entropy. Methods in Enzymology, 384, 172–184.
Richman, J. S., & Moorman, J. R. (2000). Physiological time series analysis using approximate entropy and sample entropy. American Journal of Physiology-Heart and Circulatory Physiology, 278(6), H2039–H2049.
Roberts, Timothy P. L., Khan, Sarah Y., et al. (2010). MEG detection of delayed auditory evoked responses in autism spectrum disorders: Towards an imaging biomarker for autism. Autism Research, 3(1), 8–18.
Rubenstein, J. L., & Merzenich, M. M. (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behaviour, 2(5), 255–267.
Russo, N., Foxe, J. J., et al. (2010). Multisensory processing in children with autism: High-density electrical mapping of auditory-somatosensory integration. Autism Research, 3(5), 253–267.
Rutter, M., Bailey, A., et al. (2003). SCQ: Social Communication Questionnaire. Los Angeles: Western Psychological Services.
Sakkalis, V. (2011). Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Computers in Biology and Medicine, 41(12), 1110–1117.
Schmitz, N., Rubia, K., et al. (2006). Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry, 59(1), 7–16.
Schultz, R. T., Gauthier, I., et al. (2000). Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry, 57(4), 331–340.
Sheikhani, A., Behnam, H., et al. (2012). Detection of abnormalities for diagnosing of children with autism disorders using of quantitative electroencephalography analysis. Journal of Medical Systems, 36(2), 957–963.
Simmons, D. R., Robertson, A. E., et al. (2009). Vision in autism spectrum disorders. Vision Research, 49(22), 2705–2739.
Sporns, O., Tononi, G., et al. (2000). Connectivity and complexity: The relationship between neuroanatomy and brain dynamics. Neural Network, 13(8–9), 909–922.
Sporns, O., & Zwi, J. D. (2004). The small world of the cerebral cortex. Neuroinformatics, 2(2), 145–162.
Takahashi, T., Cho, R. Y., et al. (2010). Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: A multi scale entropy analysis. Neuroimage, 51(1), 173–182.
Tononi, G., Sporns, O., et al. (1994). A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proceedings of the National Academy of Sciences, 91(11), 5033–5037.
Tsiaras, V., Simos, P. G., et al. (2011). Extracting biomarkers of autism from MEG resting-state functional connectivity networks. Computers in Biology and Medicine, 41(12), 1166–1177.
Vissers, M. E., Cohen, M. X., et al. (2012). Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and Biobehavioral Reviews, 36(1), 604–625.
Wechsler, D. (2003). Wechsler Intelligence Scale for Children (3rd ed.). San Antonio: The Psychological Corporation.
Weng, S. J., Wiggins, J. L., et al. (2010). Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research, 1313, 202–214.
Wiggins, J. L., Peltier, S. J., et al. (2011). Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Research, 1380, 187–197.
Williams, D. L., Goldstein, G., et al. (2006). Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychol, 12(4–5), 279–298.
Yum, M. K., Jung, K. Y., et al. (2008). Effect of a ketogenic diet on EEG: Analysis of sample entropy. Seizure, 17(6), 561–566.
Zhou, D., Thompson, W. K., et al. (2009). MATLAB toolbox for functional connectivity. Neuroimage, 47(4), 1590–1607.
Acknowledgments
This work is supported in part by NIH R01DC008871 (PI: T. Roberts) and NIH P30HD026979 (Neuroimaging Core, Director: T. Roberts), NIH MH092862 (PI: R. Verma) and NIH MH098010 (PI: R. Verma), Pennsylvania Department of Health SAP # 4100042728 and SAP # 4100047863 (PI: R. Schultz). Dr. Roberts would like to thank the Oberkircher Family for the Oberkircher Family Chair in Pediatric Radiology at the Children’s Hospital of Philadelphia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ragini Verma and Timothy P. L. Roberts are joint senior authors.
Appendices
Appendix 1: Computation of Multi-Scale Entropy
Let \( {\mathbf{x}}_{N} (t_{i} ) = \left[ {x(t_{i} ),x(t_{i} + T),\ldots ,x(t_{i} + (N - 1)T)} \right] \) represent a vector of length N starting at time point t i of a signal (or a time series) recorded by the sampling period T. SE is defined as the logarithmic conditional probability that two similar sequences of length m, i.e. x m (t p ) and x m (t q ), remain similar when added one sample to their length, i.e. x m+1 (t p ) and x m+1 (t q ). This can be quantified as follows
where \( n_{i}^{m} (r) \) is the number of m-length vectors x m (t j ) which are within a distance of r to x m (t i ) when self matches are not counted (Richman and Moorman 2000; Richman et al. 2004; Costa et al. 2005). The distance based on which the similarity between the vectors x m (t i ) and x m (t j ) are calculated is defined as the maximum absolute difference between their elements (Costa et al. 2005). Figure 10 illustrates how this SE is calculated.
A simulated time series x(t) of 50 samples illustrating the SE calculation when m = 2 and r = 0.2σ x . The green, pink, and red dashed lines around the first three datapoints x(0), x(0.02), and x(0.04) represent the area of ±r around these points, respectively. Here, a data point is determined matched with the first data point, if it is within the range of x(0) ± r. All those similar to the first data point are shown by green circles, and accordingly, pink and red circles are used for those matched with the second and third data points, respectively. In the segment of time series shown here, there are two two-component templates of green-pink matched with [x(0), x(0.02)] while there is only one three-component templates of subsequent green-pink-red points similar to [x(0), x(0.02), x(0.04)]. This procedure is repeated to calculate the ration of all two- and three-component template matches, from which the SE is calculated (Color figure online)
Multi-scale entropy (MSE) is then defined as the sample entropies measured at consecutive coarse grained time series y τ corresponding to the scale τ. The coarse grained procedure is performed by
in which at each scale τ the data points are first divided into nonoverlapping segments of length τ, and then the data points inside each segment are averaged to calculate the corresponding \( y_{j}^{\tau } \). Figure 11 illustrates the coarse grained time series at the scale 3.
By computing SE at various scales, MSE curves can be generated and used to compare the relative complexity of the power-normalized time series. The minimum, maximum, or average of the MSE measures along the scales can be used as different scalar measures of complexity. The two parameters m and r in MSE need to be set for proper analysis. It has been shown (Costa et al. 2005; Takahashi et al. 2010; Bosl et al. 2011; Catarino et al. 2011) that a good statistical validity of MSE is achieved when m = 1 or 2 and r = 0.1σ – 0.25σ where σ represents the SD of the time series at scale τ = 1 (i.e. SD of the original time series). In this work, we empirically chose m = 2 and r = 0.2σ after investigating different values. This procedure is performed for each sensor and each frequency band. A higher MSE value implies lower repeatability and accordingly higher signal complexity.
Appendix 2: Synchronization Likelihood
The time–frequency SL technique assumes that two signals are synchronized if a pattern of one signal repeats itself at certain time instants for a number of times within a certain period and another pattern in the other signal repeats itself at those same time instants (Montez et al. 2006).
For a given signal at channel k, i.e. x k (t), the signal pattern at any given time instant t i can be represented by an embedding vector \( \mathbf{x}_{{k,t_{i} }} = \left[ {x_{k} (t_{i} ),x_{k} (t_{i +l} ), \ldots ,x_{k} (t_{i + (m - 1)l} )} \right] \) where l is the lag and m is the length of the embedding vector. l and m are typically set to \( l = \frac{{f_{s} }}{{3h_{f} }} \) and \( m = \frac{{3h_{f} }}{{l_{f} }} + 1 \) where f s is the sampling frequency, and h f and l f are the high and low frequency contents of the signal, respectively. At each time instant t i , the Euclidean distance is then measured between the reference embedding vector \( {\mathbf{x}}_{{k,t_{i} }} \) and the set of all other embedding vectors at times t j , i.e. \( {\mathbf{x}}_{{k,t_{j} }} \), where t j lies in the range \( t_{i} - \frac{{t_{{w_{2} }} }}{2} < t_{j} < t_{i} - \frac{{t_{{w_{1} }} }}{2} \) or \( t_{i} + \frac{{t_{{w_{1} }} }}{2} < t_{j} < t_{i} + \frac{{t_{{w_{2} }} }}{2} \) where \( t_{{w_{1} }} = \frac{{2l\left( {m - 1} \right)}}{{f_{s} }} \) and \( t_{{w_{1} }} < t_{{w_{2} }} \). Then, n ref nearest embedding vectors \( {\mathbf{x}}_{{k,t_{j} }} \) are retained. This procedure is conducted for each channel k and each time instant t i . The SL between channel k 1 and channel k 2 at time instant t i is the number of simultaneous embedding vector recurrences in the two channels divided by the total number of recurrences, i.e. \( SL_{{t_{i} }} = \frac{{n_{{k_{1} k_{2} }} }}{{n_{ref} }} \). Figure 12 illustrates how SL is calculated.
An illustration of synchronization likelihood between two signals from two channels plotted by different colors. A reference pattern was selected at the time 0.074 s (thick rectangle). The recurrences in both signals are shown by thin rectangles for n ref = 10. Vertical arrows show simultaneous recurrences in both channels. SL at time 0.074 is therefore equal to the ratio between number of simultaneous recurrences and the number of n ref , i.e. SL = 5/10 = 0.5
In this work, the parameters adjustments were performed for each frequency band according to the aforementioned equations, which are shown in Table 2.
Recording from 274 sensors, we obtained a three dimensional SL matrix of 274 × 274 × T per recording per frequency, where T is the number of time instants at which \( SL_{{t_{i} }} \) is calculated. The functional connectivity matrix is then defined as the average of all the T matrices along the time instants, yielding a symmetric 274 × 274 SL matrix (per band) whose elements range between 0 and 1.
The SL connectivity matrix of each subject at each band represents a weighted graph in which each sensor represents the graph node and each SL connection between two sensors represents the graph edge weighted by their SL value. The node strength in a graph is defined as the summation of the edges between a node and all other graph nodes, i.e. \( NS_{i} = \sum\nolimits_{j = 1,j \ne i}^{274} {SL\left( {i,j} \right)} \) where NS i is the node strength of sensor i and SL(i,j) is the SL value between sensors i and j. Node strength of the connectivity graph can be interpreted as the relative strength of the corresponding node region to connect with other regions.
Rights and permissions
About this article
Cite this article
Ghanbari, Y., Bloy, L., Christopher Edgar, J. et al. Joint Analysis of Band-Specific Functional Connectivity and Signal Complexity in Autism. J Autism Dev Disord 45, 444–460 (2015). https://doi.org/10.1007/s10803-013-1915-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-013-1915-7