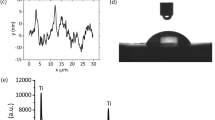
Abstract
A new strategy to render intrinsically hydrophobic microrough titanium implant surfaces superhydrophilic is reported, which is based on a rapid treatment with diluted aqueous sodium hydroxide solutions. The physicochemical characterization and protein interaction of the resulting superhydrophilic implant surfaces are presented. The superhydrophilicity of alkali treated microrough titanium substrates was mainly attributed to deprotonation and ion exchange processes in combination with a strong enhancement of wettability due to the roughness of the used substrates. Albeit these minor and mostly reversible chemical changes qualitative and quantitative differences between the protein adsorption on untreated and alkali treated microrough titanium substrates were detected. These differences in protein adsorption might account for the enhanced osseointegrative potential of superhydrophilic alkali treated microrough implant surfaces. The presented alkali treatment protocol represents a new clinically applicable route to superhydrophilic microrough titanium substrates by rendering the implant surface superhydrophilic “in situ of implantation”.








Similar content being viewed by others
References
Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Acta Orthop Scand. 1981;52:155–70.
Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–21.
Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983;49:832–7.
Albrektsson T, Wennerberg A. Oral implant surfaces: part 1—review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–43.
Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–54.
Wennerberg A, Albrektsson T, Andersson B, Krol JJ. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin Oral Impl Res. 1995;6:24–30.
Wennerberg A, Hallgren C, Johansson C, Danelli S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin Oral Impl Res. 1998;9:11–9.
Cooper LF. A role of surface topography in creating and maintaining bone at titanium endosseous implants. J Prosth Dent. 2000;84:522–34.
Davies JE. Understanding peri-implant endosseous healing. J Dent Edu. 2003;67:932–49.
Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG 63). J Biomed Mater Res. 1995;29:389–401.
Lossdörfer B, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, Cochran DL, Boyan BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res. 2004;70A:361–9.
Pilliar RM. Implant surface design for development and maintenance of osseointegration. In: Ellingson JE, Lyngstadaas SP, editors. Bio-implant interface. Boca Raton: CRC press; 2003. p. 43–58.
Franchi M, Fini M, Giavaresi G, Ottani V. Peri-implant osteogenesis in health and osteoporosis. Micron. 2005;36:630–44.
Keselowsky BG, Collard DM, Garcìa AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci USA. 2005;102:5953–7.
Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res. 2005;74A:49–58.
Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–33.
Ferguson SJ, Broggini N, Wieland M, de Wild M, Rupp F, Geis-Gerstorfer J, Cochran DL, Buser D. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J Biomed Mater Res. 2006;78A:291–7.
Kim HM, Miyaji F, Kokubo T, Nakamura T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J Biomed Mater Res. 1996;32:409–17.
Takadama T, Kim HM, Kokubo T, Nakamura T. An X-ray photoelectron spectroscopy study of the process of apatite formation on bioactive titanium metal. J Biomed Mater Res. 2001;55:185–93.
Jonášovà L, Müller F, Helebrant A, Strnad J, Greil P. Biomimetic apatite formation on chemically treated titanium. Biomaterials. 2004;25:1187–94.
Vroman L. The life of an artificial device in contact with blood: initial events and their effect on its final state. Bull N Y Acad Med. 1988;64:352–7.
Olivares-Navarette R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. Integrin α2β1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci USA. 2008;105:15767–72.
Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127:8168–73.
Vroman L, Adams AL, Fischer GC, Munoz PC. Interaction of high molecular weight kininogen in plasma at interfaces. Blood. 1980;55:156–9.
Textor M, Sitting C, Frauchiger V, Tosatti S, Brunette DM. Properties and biological significance of natural oxide films on titanium and its alloys. In: Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in medicine. Berlin: Springer; 2001. p. 172–224.
Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–75.
Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res. 2006;76A:323–34.
Bico J, Thiele U, Quéré D. Wetting of textured surfaces. Colloids Surf A Physicochem Eng Aspect. 2002;206:41–6.
Ameen AP, Short RD, Johns R, Schwach G. The surface analysis of implant materials. The surface composition of a titanium dental implant material. Clin Oral Impl Res. 1993;4:144–50.
Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, Textor M, Spencer ND, Brunette DM. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. J mater sci mater med. 2002;13:535–48.
Rupp F, Scheideler L, Rehbein D, Axmann D, Geis-Gerstorfer J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 2004;25:1429–38.
Aita H, Hori N, Takeuchi M, Suzuki T, Yamada M, Anpo M, Ogawa T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009;30:1015–25.
Baier RE, Meyer AE. Future directions in surface preparation of dental implants. J Dent Ed. 1988;52:788–91.
Feng B, Weng J, Yang BC, Qu SX, Zhang XD. Characterization of surface oxide films on titanium and adhesion of osteoblasts. Biomaterials. 2003;24:4663–70.
Jennissen HP. Ultra-hydrophilic transition metals as histophilic biomaterials. Macromol Symp. 2005;225:43–69.
Von Wilmowsky C, Müller L, Lutz R, Lohbauer U, Rupp F, Neukam FW, Nkenke E, Schlegel KA, Müller FA. Osseointegration of chemically modified titanium surfaces: an in vivo study. Adv Eng Mater. 2008;10:B61–6.
Stadlinger B, Lode A, Eckelt U, Range U, Schlottig F, Hefti T, Mai R. Surface-conditioned dental implants: an animal study on bone formation. J Clin Periodont. 2009;36:882–91.
Stadlinger B, Mai R, Lode AT, Eckelt U. Evaluation of surface conditioned dental implants–an animal study. Int J Oral Maxillofac Surg. 2009;38:454.
Whitehouse DJ. Handbook of surface metrology. London: Institute of Physics Publishing; 1994.
Wieland M, Textor M, Spencer ND, Brunette DM. Wavelength-dependent roughness: a quantitative approach to characterizing the topography of rough titanium surfaces. Int J Oral Maxillofac Implants. 2001;16:163–81.
McCafferty E, Wightman JP. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf Interface Anal. 1998;26:549–64.
Lim YJ, Oshida Y. Initial contact angle measurements on variously treated dental medical titanium materials. Bio-Med Mater Eng. 2001;11:325–41.
Connor PA, Dobson KD, McQuillan AJ. Infrared spectroscopy of the TiO2/aqueous solution interface. Langmuir. 1999;15:2402–8.
Schliephake H, Scharnweber D. Chemical and biological functionalization of titanium for dental implants. J Mater Chem. 2008;18:2404–14.
Boehm HP. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss Faraday Soc. 1971;52:264–75.
Nygren H, Stenberg H, Karlsson C. Kinetics supramolecular structure and equilibrium properties of fibrinogen adsorption at liquid-solid interfaces. J Biomed Mater Res. 1992;26:77–91.
Nygren H. Initial reactions of whole blood with hydrophilic and hydrophobic titanium surfaces. Coll Surf B Bioint. 1996;6:329–33.
Michael KE, Vernekar VN, Keselowsky BG, Meredith JC, Latour RA, García AJ. Adsorption–induced conformational changes in fibronectin due to interactions with well-defined surface chemistries. Langmuir. 2003;19:8033–40.
Scheideler L, Rupp F, Wieland M, Geis-Gerstorfer J. Storage conditions of titanium implants influence molecular and cellular interactions; 83rd general session and exhibition of the international association for dental research; 2006. Poster # 870.
Salzman EW, Linden J, McManama G, Ware JA. Role of fibrinogen in activation of platelets by artificial surfaces. Annals NY Acad Sci. 1987;516:184–95.
Tang L, Wu Y, Timmons RB. Fibrinogen adsorption and host tissue responses to plasma functionalized surfaces. J Biomed Mater Res. 1998;42:156–63.
Palmquist A, Jarmar T, Emanuelsson L, Brånemark R, Engqvist H, Thomsen P. Forearm bone-anchored amputation prosthesis: A case study on the osseointegration. Acta Orthop. 2008;79:78–85.
Serro AP, Fernandes AC, Saramago BJ. Calcium phosphate deposition on titanium surfaces in the presence of fibronectin. Biomed Mater Res. 2000;49:345–52.
Acknowledgments
The authors are grateful to Prof. Uwe Pieles and Theo Bühler (FHNW Muttenz, Switzerland) for technical support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tugulu, S., Löwe, K., Scharnweber, D. et al. Preparation of superhydrophilic microrough titanium implant surfaces by alkali treatment. J Mater Sci: Mater Med 21, 2751–2763 (2010). https://doi.org/10.1007/s10856-010-4138-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4138-x